I was asked why the regulators would go along with ending their regulatory role and accepting the decision re drug licensure made elsewhere
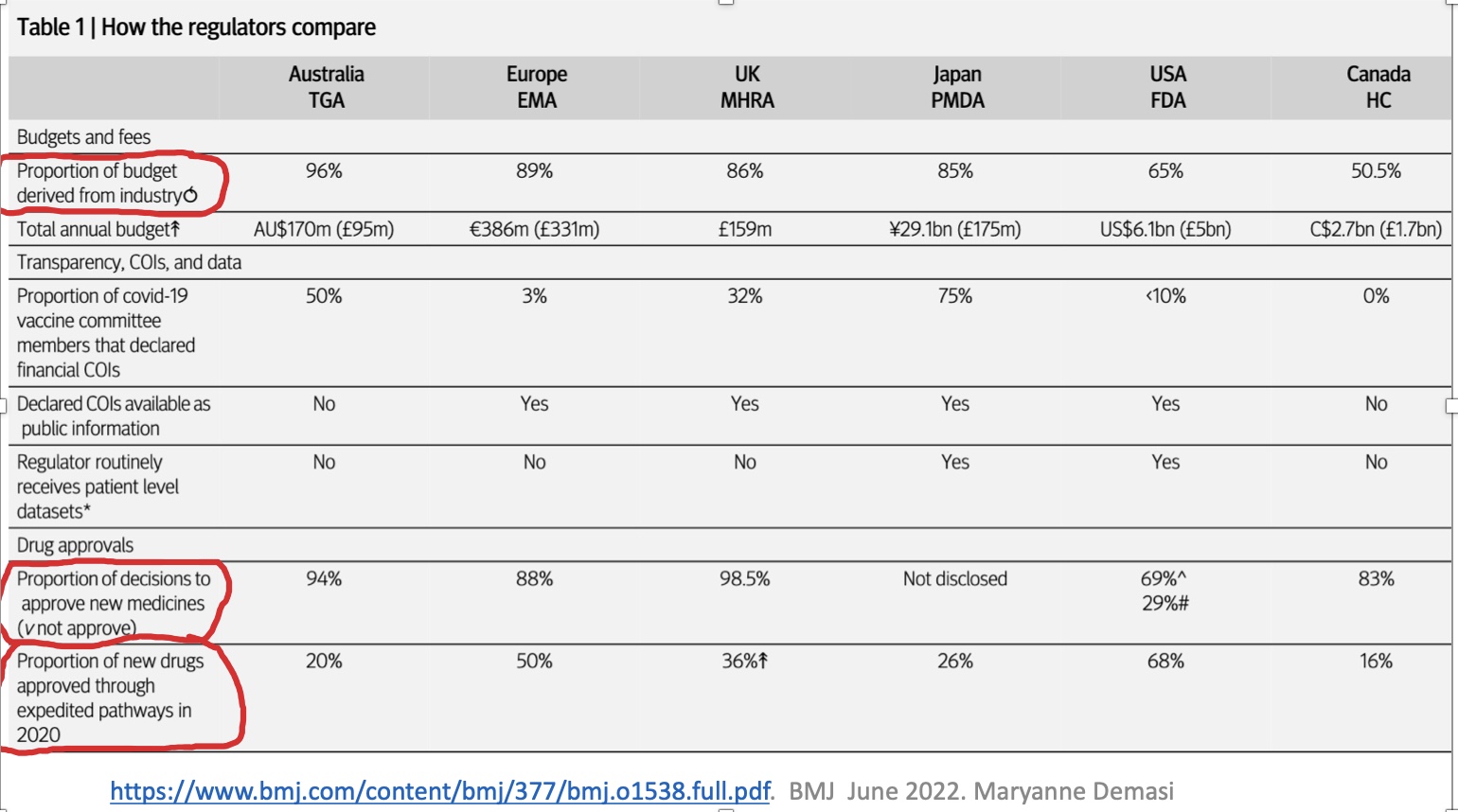
The answer is that they stopped regulating a long time ago. Here is an example from today, one of many. Their funders make the decisions, not the regulators.
Sarepta is extremely expensive and probably does not work. However it got licensed with a purchased 6 month review. The mfr must pay extra to get a decision, often before the staff have read all the documents.
https://www.fiercebiotech.com/biotech/fdas-marks-i-contradict-fda-reviewers-trepidation
“… the FDA granted accelerated approval to Sarepta’s gene therapy for four and five-year-old patients with Duchenne muscular dystrophy. The decision was at odds with agency reviewers who said ahead of an advisory meeting to discuss the treatment that the available data did not provide “unambiguous evidence” of clinical benefit.
Marks wrote in a memo after the fact that he disagreed with the reviewers’ assessment of the efficacy in that age population and came to “a different conclusion.”‘
Why does Marks overrule his expert committee when he was a cancer doctor and neither a neurologist or musculoskeletal expert or clinical trials expert? Here’s why: