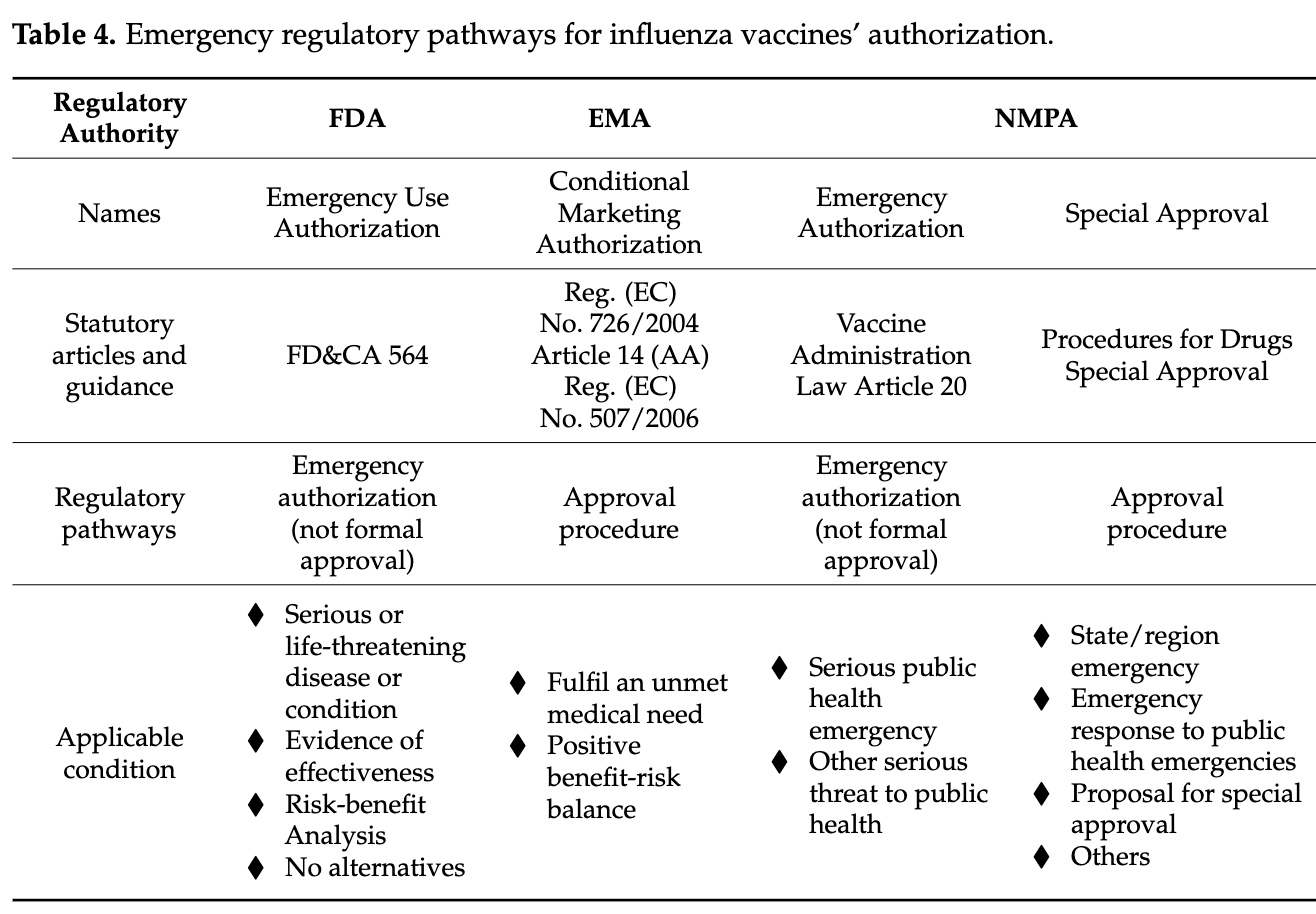
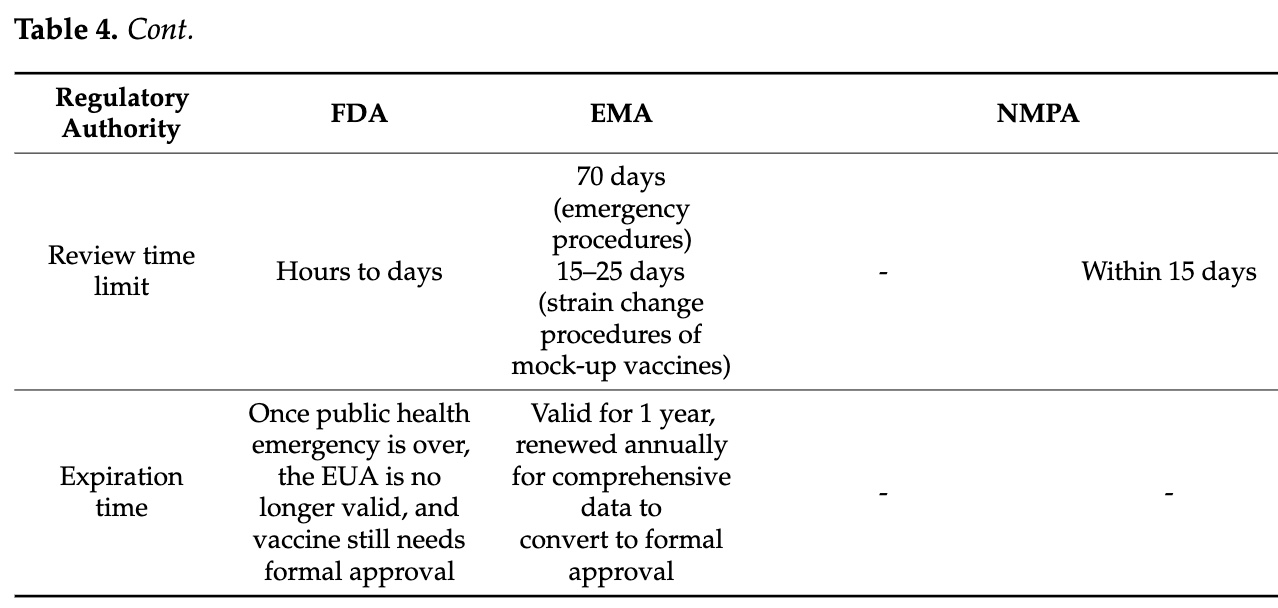
The BMGF funded a comprehensive review of emergency authorization procedures for pandemic vaccines. Forewarned is forearmed.
We must thank BMGF for laying out the wily ways our governments avoid truly regulating emergency vaccines. Bill Gates wanted this information, and so do we.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10610648/pdf/vaccines-11-01573.pdf
So they junked the term “mock-up vaccine” and replaced it with pre-pandemic vaccines or pandemic preparedness influenza vaccines. Much better terminology to use to hide the fact your mock-up vaccine is just a pretend vaccine.
Note that these pre-vaccines “normally contain a strain of bird flu virus that few people in the world have already been exposed to…” This means the mock-up CANNOT be tested for efficacy since the infectious virus is not circulating. There is no population in which to test it. (If it was circulating, it would be a pandemic virus, not a pre-pandemic virus.)
While FDA uses antibody levels as a “surrogate marker” of efficacy, that is a bogus procedure that does not produce valid results. Until recently, FDA knew that (according to its own rules) surrogate markers had to be validated, but when COVID hit, that basic knowledge was thrown out the window. See what FDA itself had to say:
“Before a surrogate endpoint can be accepted in place of a clinical outcome, extensive evidence must accumulate, including evidence from epidemiological studies and clinical trials. Usually clinical trials are needed to show that the surrogate endpoint can be relied upon to predict, or correlate with, clinical benefit in a context of use. Surrogate endpoints that have undergone this extensive testing are called validated surrogate endpoints and these are accepted by the FDA as evidence of benefit.”
FDA currently ignores this shibboleth in its regulation of prepandemic/pandemic vaccines.
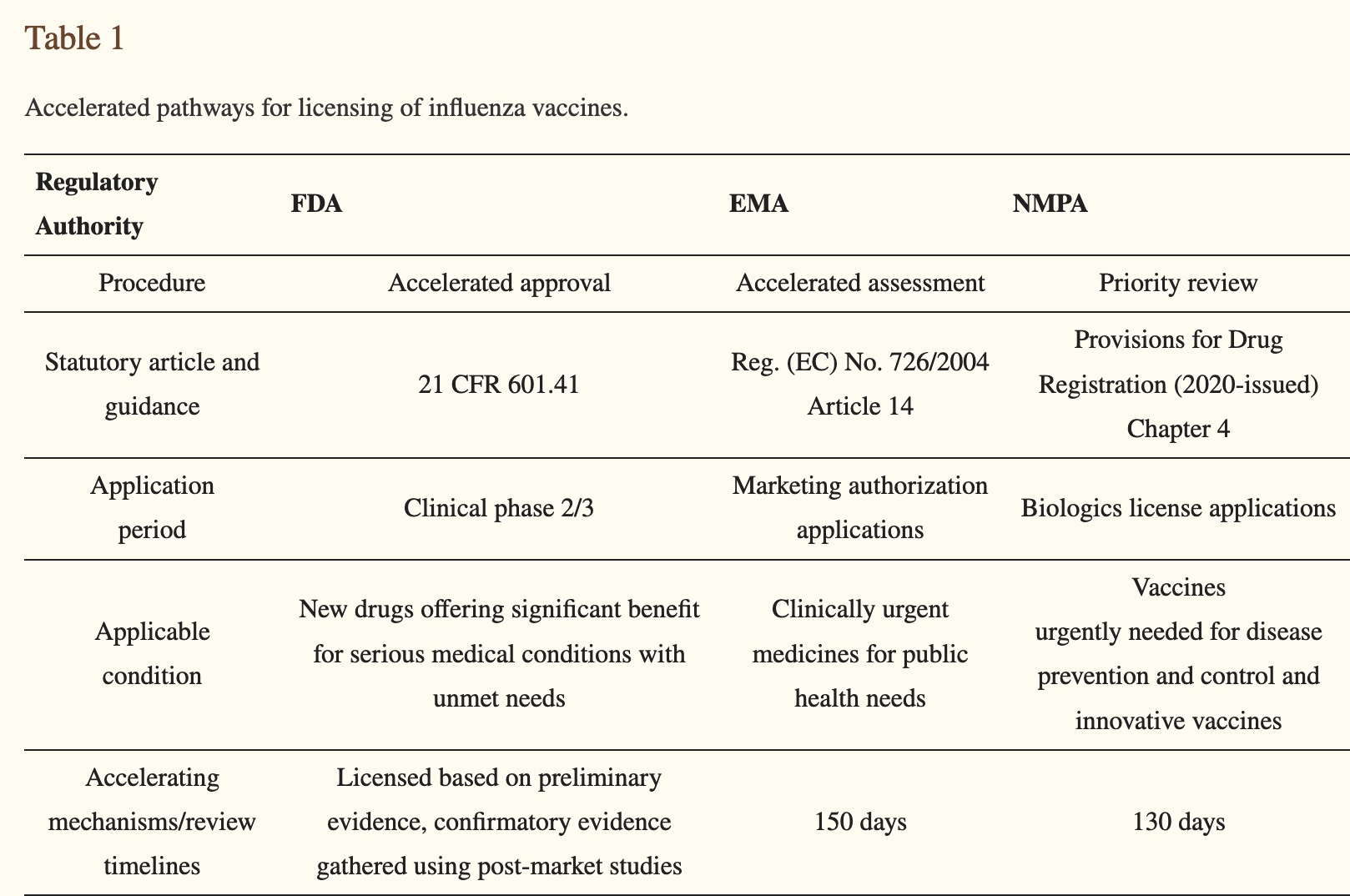
“Accelerated and flexible procedures” are what injured or killed millions of humans as a result of the fraudulently tested and fraudulently authorized and approved COVID vaccines. What worries me is that the regulators plan to do exactly the same thing again. After all, it takes a long time to issue rules and regulations, and I’m sure it wasn’t easy to get the PREP Act through.
Let’s look at some more useful information in this paper. Most of the paragraph below I included above, but am adding it here so the entire section is in one place.
Above, the method of the mock-up is clarified expeditiously. This explains the 5 days for the EMA to approve GSK’s Pandemrix in 2009.
The above explains why 3 mock-up vaccines are already FDA-licensed for bird flu: so that any of their platforms can be used in a hurry. Note they do not use the words “efficacy” or “effectiveness” since the mock-ups were not tested to see if they work. The term “immunogenicity” simply means the vaccine induces antibodies. Which all vaccines do. Sometimes they are effective, sometimes not, and sometimes they induce a more severe disease. Effectiveness was “inferred”, i.e., surmised, based on the fact that seasonal flu vaccines seem to work. Though some years they don’t.
Who knew? The FDA licensed 4 pre-pandemic H1N1 vaccines, grandfathered in based on the licenses of non-pandemic, seasonal flu vaccines. No clinical trials. Think of the $ savings for the manufacturers.
Great! You find out if it works after you roll it out to the entire population, and once everyone has received, you learn its safety and efficacy. Or, like the COVID vaccines, you never learn either because the federal government sponsored “The Science” to get its preordained conclusions, and furthermore censored or retracted scientific studies that challenged the scientific narrative.
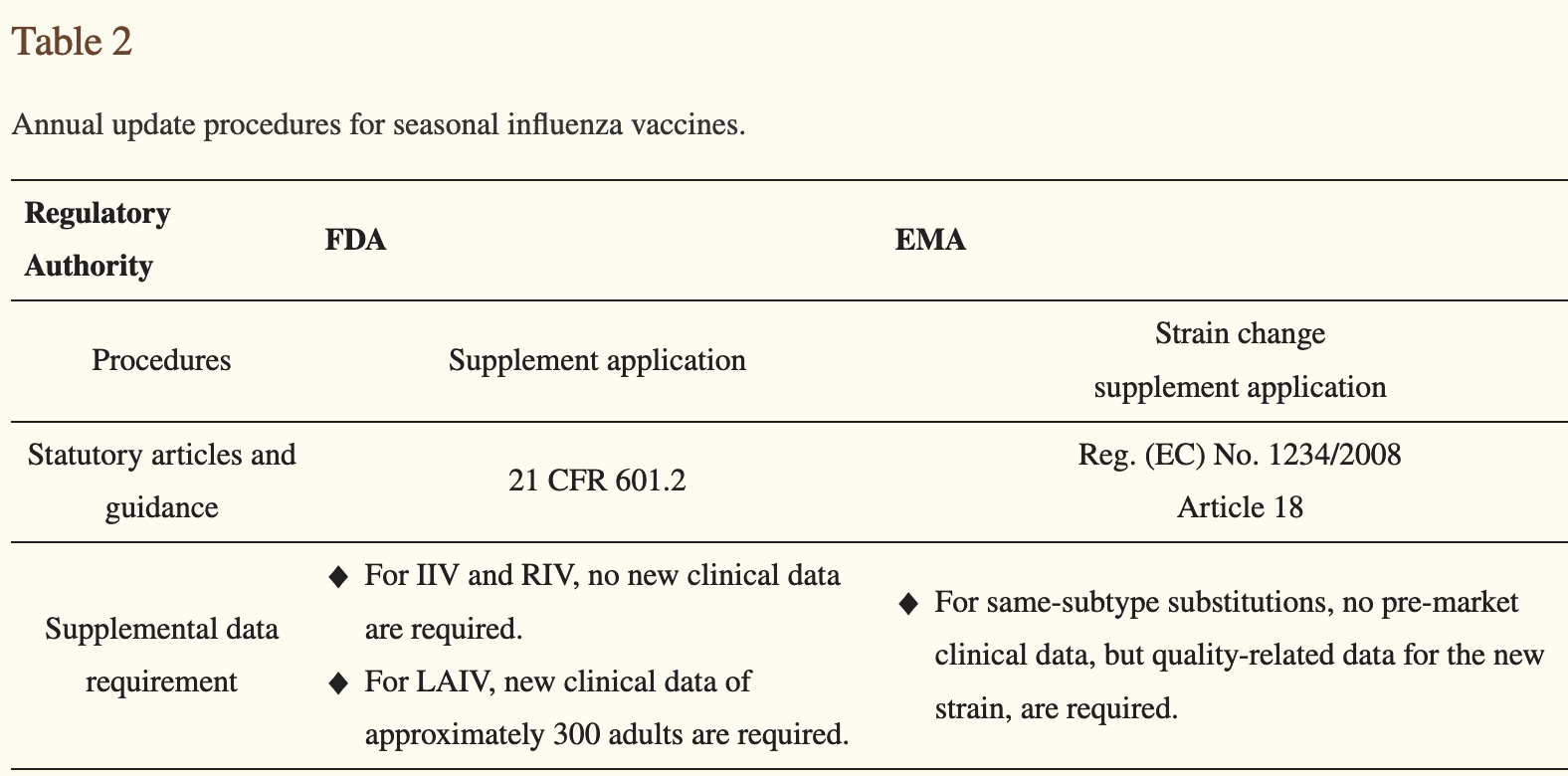
IIV= inactivated influenza vaccine and RIV=recombinant influenza vaccine. No clinical (human) data required for each year’s flu shot. Do you still want to go get them?
The US (and probably the EMA) have two pathways by which untested or minimally tested or fraudulently tested vaccines can be rolled out:
-
the PREP Act process which authorizes vaccines
-
the mock-up process which licenses them.
Either or both can be used to fool us at the last minute. The FDA is already approving (licensing) mRNA COVID vaccines with its grandfathering-in process, based on the so-called efficacy and safety of earlier mRNA COVID vaccines adapted to different strains, essentially the “mock-up” procedure.
Caveat Emptor. Let the buyer beware. Especially when it is “free” and even more so when you are offered some benefit for rolling up your sleeve. Forewarned is forearmed. Please share.