Why are COVID boosters being grandfathered in without testing required for all other vaccines–except flu shots?
Failing to test vaccines before rolling them out is not the fallback position, but the plan
Why should we have to show our COVID vaccines are effective? say Pharma and STAT. Why not go with precedent (the grandfathering in of new flu shots each year) ?
Here is a snippet from today’s STAT:
“Makary’s vaccine playbook at FDA reads like RFK Jr.’s”
When it comes to vaccine criticism, it appears both FDA Commissioner Marty Makary and Health Secretary Robert F Kennedy Jr. are on the same page. Never mind the hopes public health experts once expressed, believing that as a physician-scientist, Makary would protect the agency from the broader vaccine skepticism circulating in the Trump administration. Last weekend Makary signaled that the FDA may depart from longstanding precedent by requiring Covid vaccine makers to submit new effectiveness data before adjusting their products for new strains, potentially hobbling efforts to protect people from evolving disease.
Like Kennedy, Makary has downplayed the seriousness of the current measles outbreak while saying he supports the MMR vaccine. He also decried “absolutism” in the vaccine schedule. He didn’t defend ousted vaccine regulator Peter Marks. And information from the HHS media office directed FDA officials to add “the decision to vaccinate is a personal one” when responding to a journalist’s question about how the agency monitors safety.
Who reads STAT? People who don’t have to pay for it themselves. Industry folks. The subscription costs are steep, it does include some advertising aka advocacy articles, and there are only a few articles published daily. In other words, it is an industry rag, though it is not designed to look like one. Virtually everything (except sponsored articles?) is behind a paywall. I had to say that so you can read STAT with the correct mindset.
STAT does have 2 journalists who had excellent reputations: Ed Silverman and Helen Branswell. Helen can investigate, but she is very pro-industry and no longer balanced. She has singlehandedly covered much of the bird flu story. Good facts, bad spin. STAT’s other writers are generally not worth reading.
What is the “longstanding precedent” that STAT is afraid the FDA may depart from? It is grandfathering in vaccines for approval without efficacy data and with only a smidgen of (inadequate) safety data.
Flu shots take a minimum of 6 months to develop and manufacture. Efforts to speed up the process for 13 years have so far failed. There are no mRNA flu shots on the market (yet, anyway). Many production methods have been tried, and flu shots have been used (as R. Malone and I have said before) to test out various adjuvants, amounts of antigen, and various production platforms on tens of millions of unwary subjects. Each year there are up to a dozen different flu shots offered, though your local drugstore probably only stocks a couple.
The choice of antigens that go into the shots is somewhat arbitrary, based on the strains circulating during the prior year and strains circulating in the southern hemisphere about 6 months before the northern hemisphere flu season. FDA, using US advisers and those associated with the WHO, reviews worldwide strain data, and picks the strains to be used in that year’s vaccines. CDC then tells manufacturers how many doses it will buy for the Medicare, Medicaid, and Vaccines for Children programs, which are provided free to 61 US jurisdictions (states, territories, Indian Health Service).
The seasonal influenza vaccines become available in late summer or early fall, and are almost immediately pushed out to the public. If you must have one, I advise you wait until around December 1 to get it, as any immunity wears off quickly, and flu season in the US really starts in late December. It takes about 2 weeks to generate high antibody levels.
Efficacy varies from year to year. Last year, they say efficacy was -27%. That means if you were vaccinated you were 27% more likely than an unvaccinated person to get a clinical case of flu. CDC says efficacy averages about 40%. Cochrane experts have said you need to vaccinate roughly 70 people, on average, to prevent one case of flu. I have covered all this in more detail in previous years.
While the grandfathering in of flu shots is problematic in my view, it has been occurring for a very long time—longer than the 25 years I have observed it. It does not meet FDA’s normal standards for vaccine licensure. How legal is it?
Ignoring the legal question, public health officials decided they could apply the flu model to grandfather in COVID vaccines and bird flu vaccines, without testing them in humans in any meaningful way before rolling them out.
For COVID the federal agencies encouraged bogus clinical trials for the first vaccine iterations, and then grandfathered in all the boosters. They didn’t know if they could get away with it, since COVID was not caused by influenza viruses and there was no legal structure to support this. But so far, they have gotten away with it.
The COVID manufacturers hoped to keep up this sham, because human clinical trials are the most expensive part of vaccine development. And many vaccines fail during trials. No trials = no failures. And STAT has the nerve to suggest that asking for evidence that the next COVID vaccine works is “potentially hobbling efforts to protect people from evolving disease.” Duh.
Since 2003, public health officials in the US and Europe have planned to pull out the “grandfathering in” scam for bird flu vaccines for humans. I have written about this before, but not for awhile. Now I want to show you screenshots from a Georgetown University webinar on bird flu vaccines that was conducted in November 2024.
The panelists show us their plans for how untested vaccines can be pushed on the population. The colored markings are my additions.

Arnold Monto gave the talk above. Nearly 90, he chaired most of the FDA advisory meetings for COVID vaccine approvals and authorizations, using an iron hand to control the discussions. Here he notes that you can’t stockpile vaccines for a pandemic cause you don’t know what is causing the pandemic till it happens. If you want a licensed vaccine, you can use “mock-up” dossiers, which were used in 2009 for the swine flu pandemic that was mild.
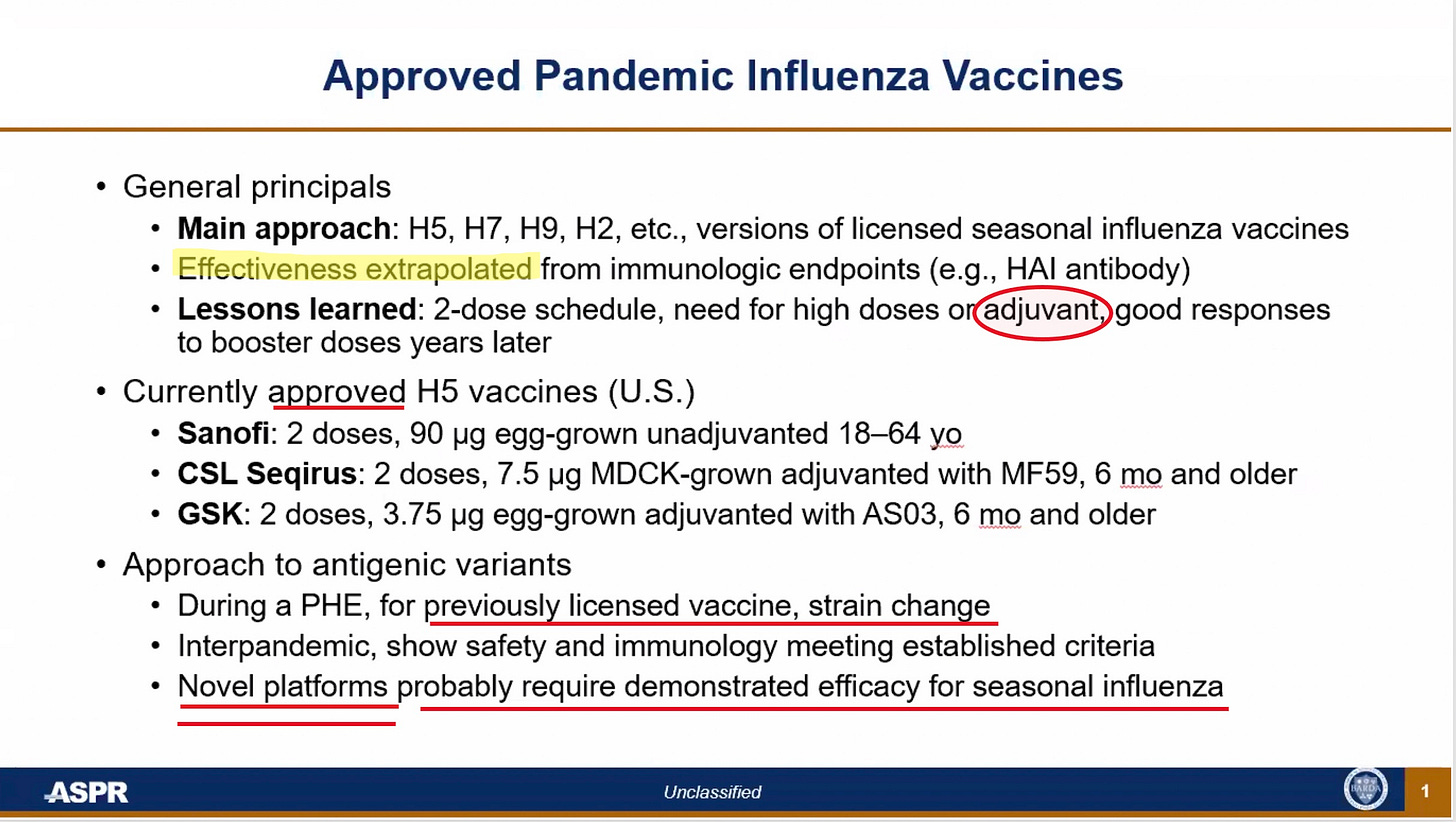
ASPR refers to the office of the HHS Assistant Secretary for Preparedness and Response. This speaker acknowledged they don’t have data on effectiveness, so they fudge it by pretending that antibody levels are an acceptable surrogate—without ever proving they are. This is technically a “no-no” for FDA but our fearless public health officials have been fine with this pretension for COVID vaccines, so why not continue it with bird flu vaccines?
3 vaccines using 3 different platforms and manufacturers have already been licensed for bird flu in the US (in 2007, 2013 and 2020). When a bird flu pandemic hits (or whenever the fear of one is sufficiently high) these platforms are used with just a strain change, and the new vaccines will be grandfathered in. This is the “mock-up” vaccine strategy.
Were a novel platform to be desired, they would actually have to test the novel vaccine.
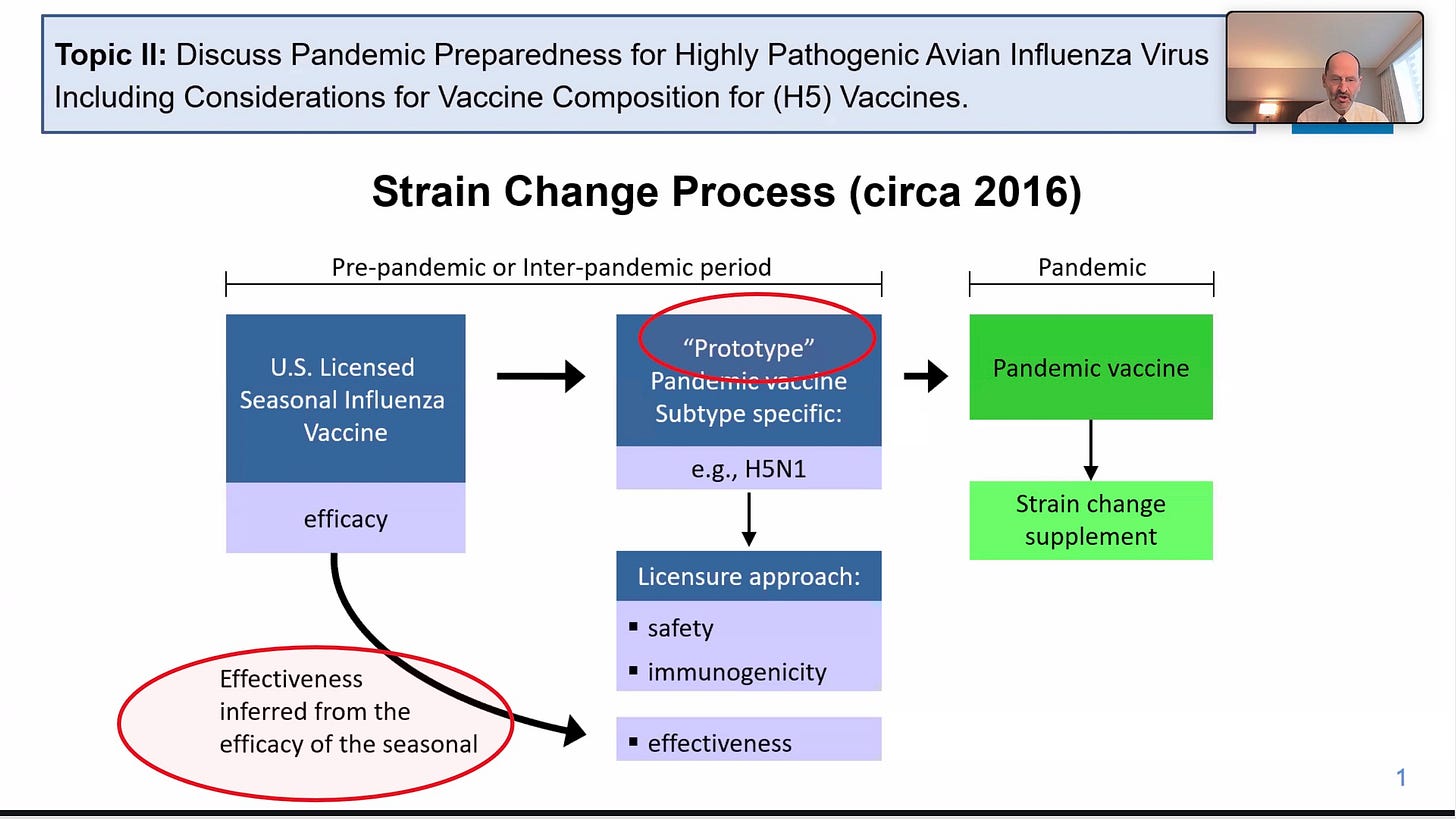
The slide above has a speaker who was very high up in FDA’s vaccine division. He makes it clear that as far as FDA is concerned, one plan is to grandfather bird flu vaccines in, based on imputing efficacy from seasonal vaccines. Sir, with a -27% efficacy last year, how is that going to work? Guess they have to infer efficacy from a different year… they are excellent at workarounds.
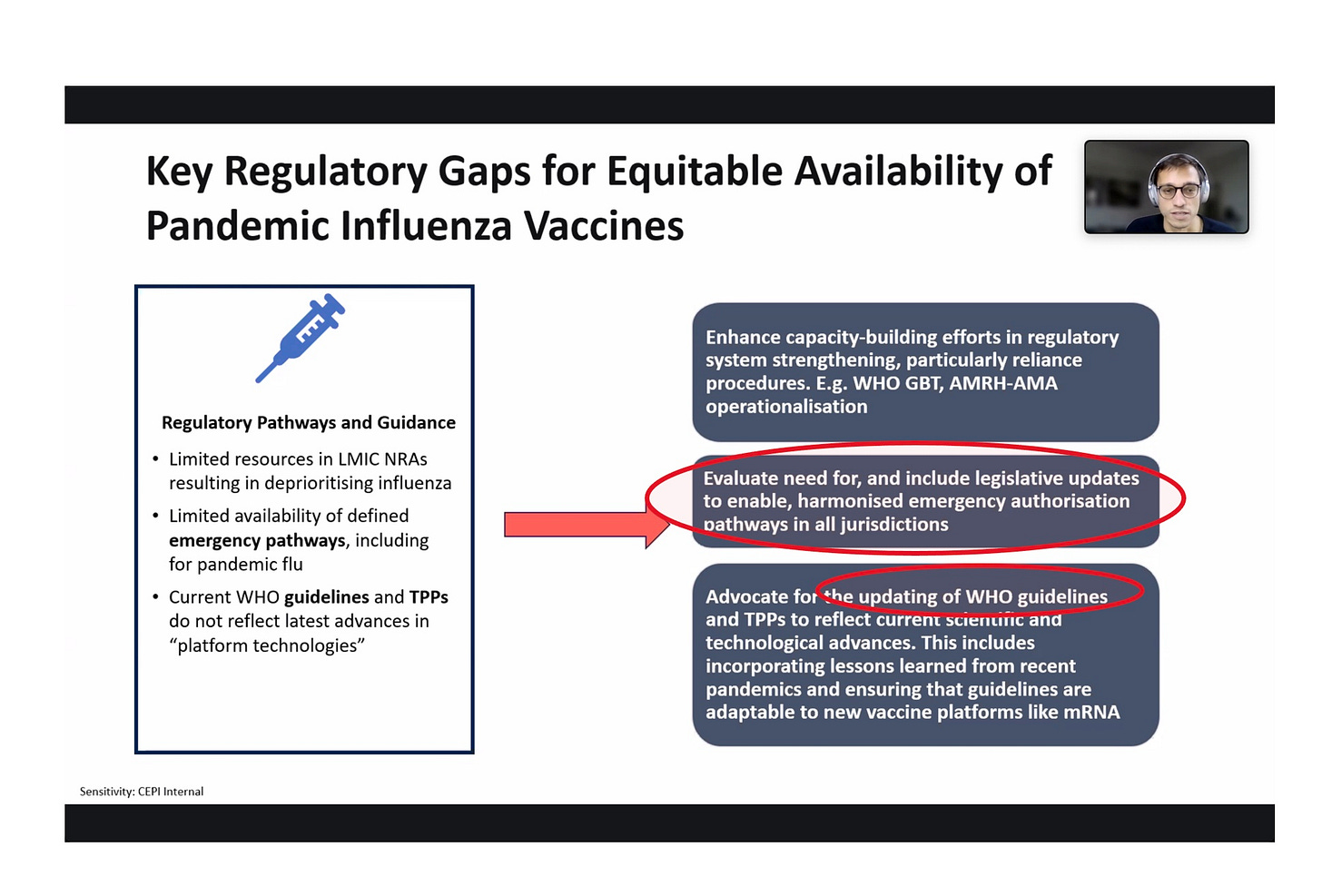
The above speaker is from CEPI. He is interested in rolling out untested vaccines to the whole world, most of which never uses flu shots or cares about flu—and which does not have a fake legal-regulatory system like the US. What to do in that case? He uses coded language to say that they hope to use the WHO and its Pandemic Treaty to force countries to legalize EUAs everywhere (“in all jurisdictions”). They definitely want mRNA vaccines to be included in whatever legal structures are adapted.
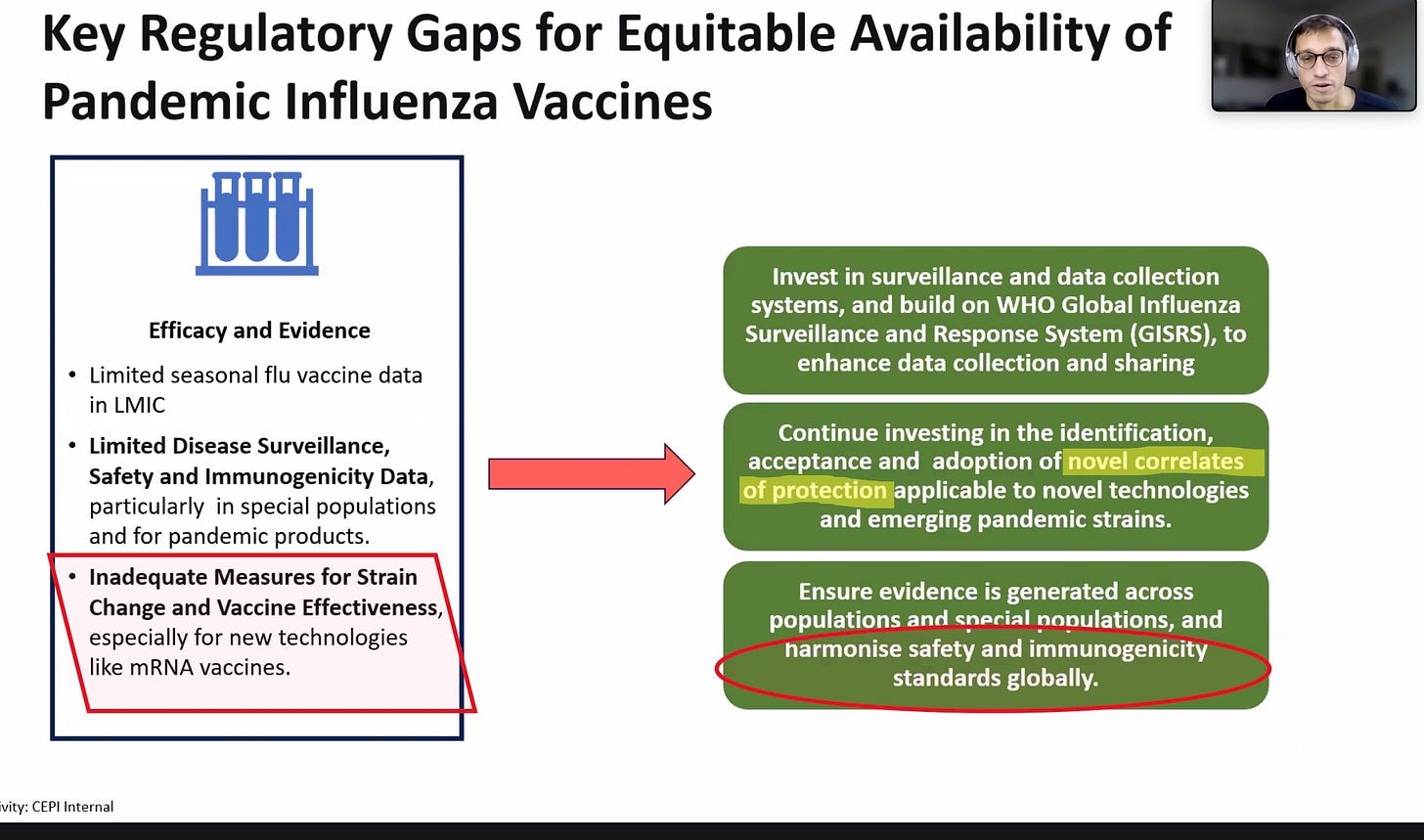
Here the CEPI speaker (founded by Bill Gates and Jeremy Farrar) admits there is no existing legal-regulatory structure in most of the world that would permit mRNA vaccines to be grandfathered in, and there are no mRNA “mock-up” vaccines for bird flu either. What to do?
Generate new bogus ways of claiming efficacy (“correlates of protection”) and make sure to harmonize whatever you do so it works in every country.
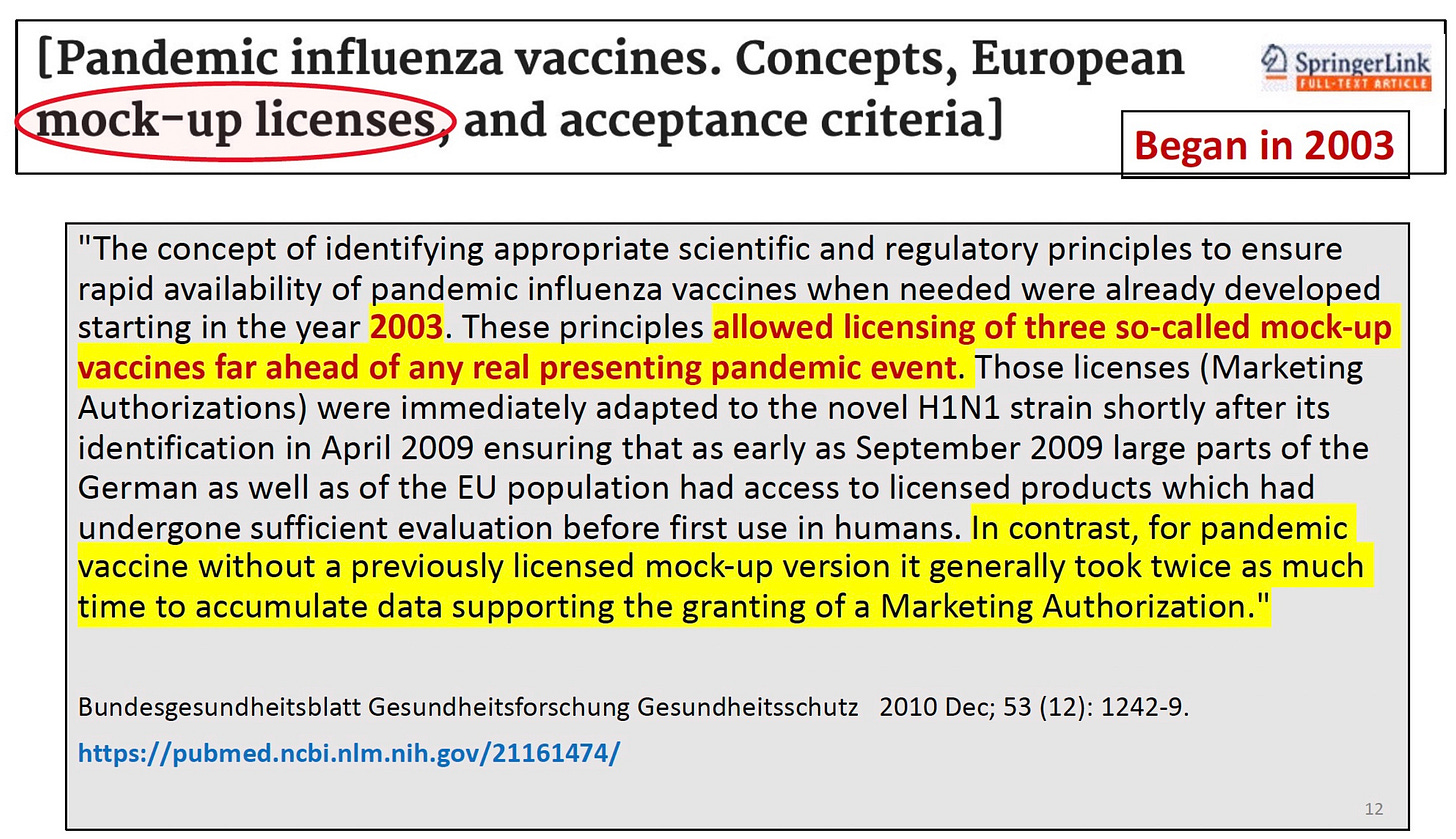
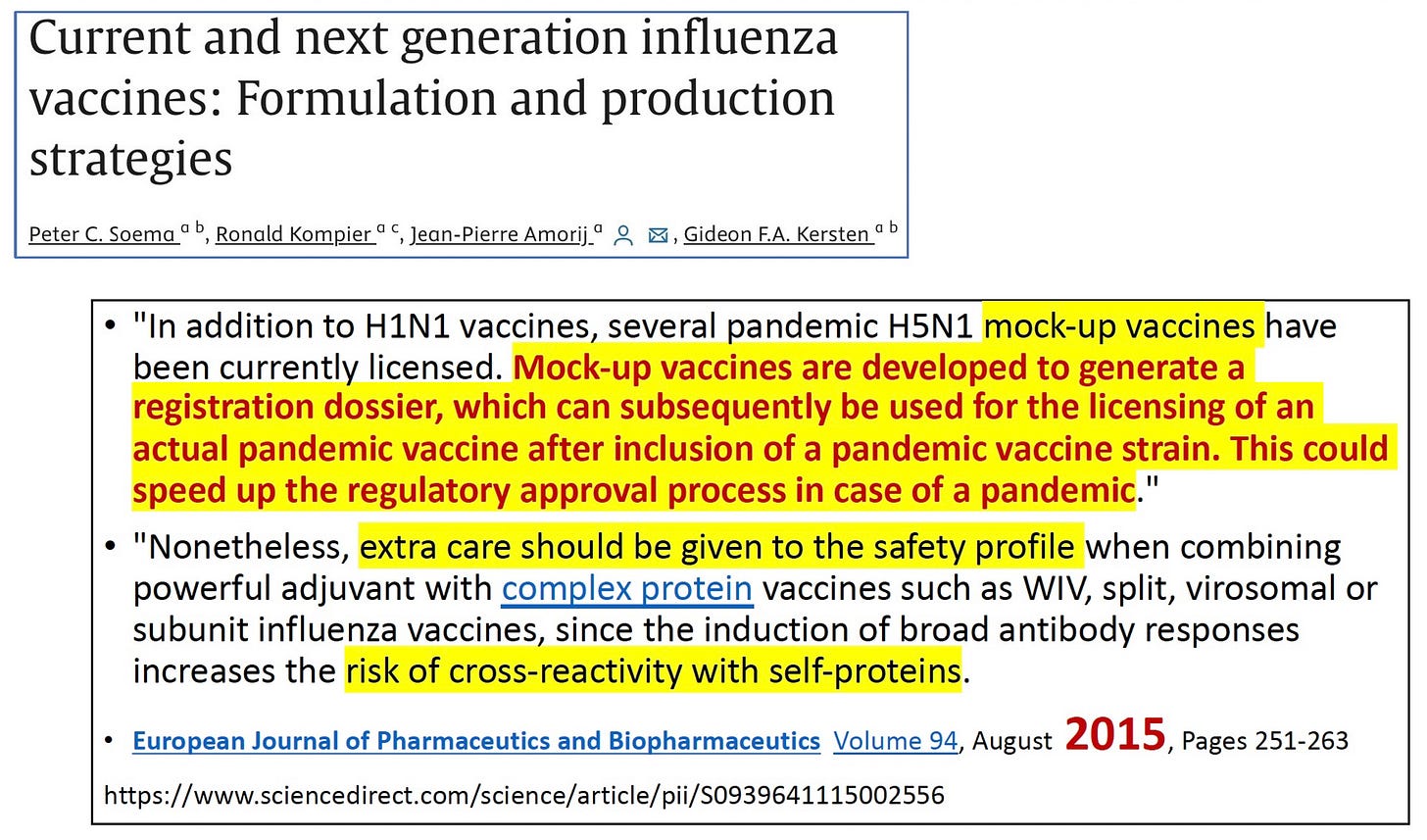
Above is an academic article published in 2010 that discusses the new concept of “mock-up” vaccines—licensing vaccines you never intend to use, so you have a paper trail whose license can be slapped onto any new antigen you want to put into the mockup framework. Yes it is a very real thing. Below is another article discussing the method from 2015.
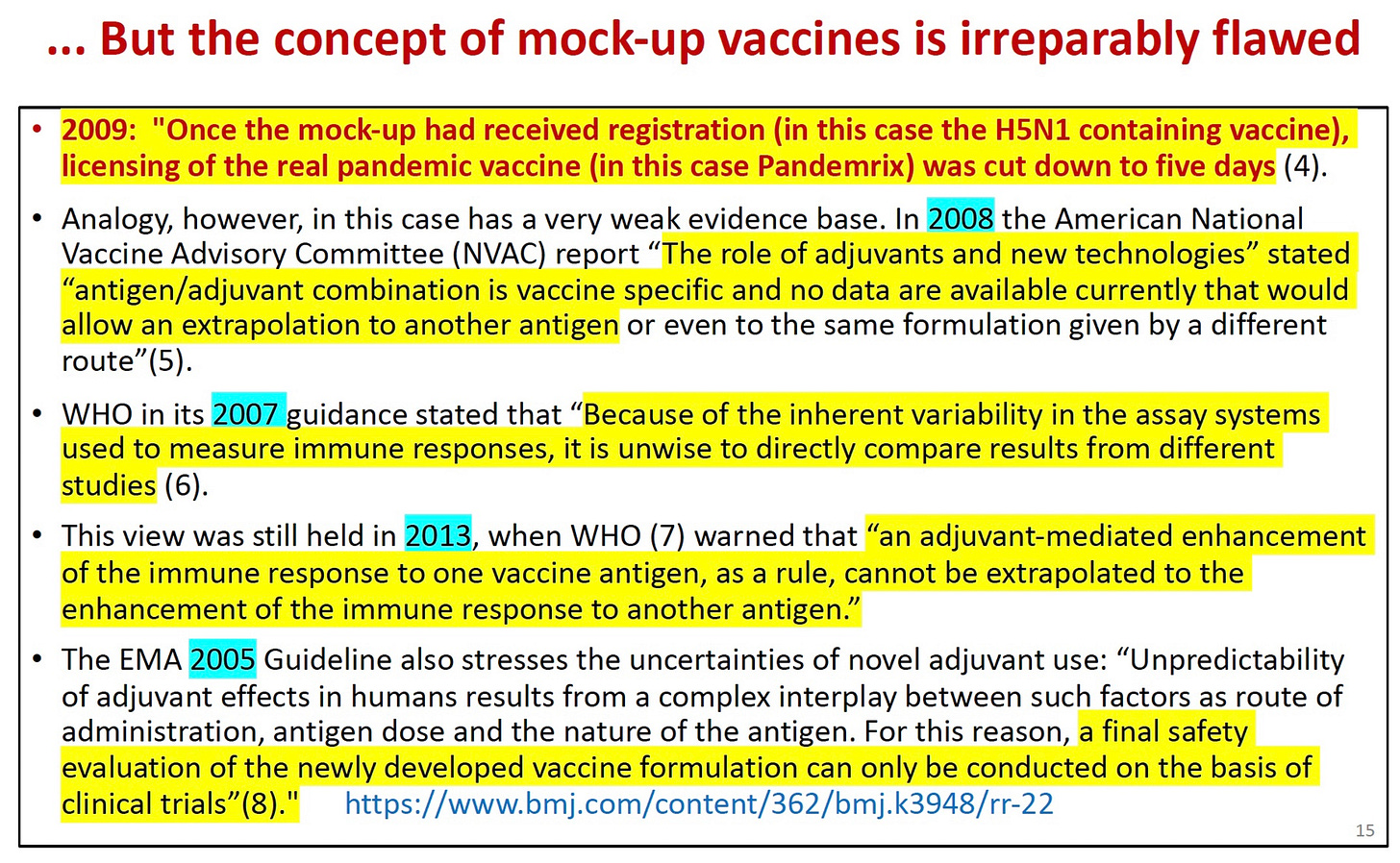
Tom Jefferson wrote a letter to the BMJ pointing out that slapping a license on an untested vaccine with a previously unused antigen was very dangerous—and many professional organizations agreed. His well-documented letter was ignored.
To sum up:

But we can stop it. Share the information. Warn your friends and relatives that untested vaccines are a feature, not a bug, of the pandemic planning agenda.
What about ivermectin and repurposed drugs? There is no money in repurposed drugs. They are not part of the plan.
ADDENDUM April 30:
More spin from STAT’s Helen Branswell today:
It’s a long time until winter respiratory disease season but May is when global vaccine experts will recommend which strains they believe Covid shots for the 2025-2026 Northern Hemisphere winter should aim to protect against. It’s not clear, writes Helen Branswell in a detailed appraisal of the outlook for U.S. vaccine policy, what, if any, involvement U.S. health agencies will have in that process, given the Trump administration’s announced withdrawal from the WHO. Even if that weren’t happening, the FDA hasn’t always parroted the WHO choice; last year its advice to manufacturers differed from what the WHO had recommended. [And according to the Cleveland Clinic that resulted in -27% efficacy.—Nass]
Many vaccine experts are concerned that HHS Secretary Robert F. Kennedy Jr. and FDA chief Marty Makary are undermining regulations, policy, and research funding that enable the creation and distribution of vaccines. Early red flags include indications that FDA won’t approve Novavax’s previously tested Covid vaccine without expensive new clinical trials, which is likely impossible, whether Covid boosters that target new strains of the virus would require lengthy testing, and whether pediatric Covid boosters will be taken off the vaccine schedule.
Grow up and smell the roses, Helen—Meryl