The horrifying FDA calculation on COVID boosters in the NEJM and the policy being proposed
Who wrote this policy and the NEJM article, signed by Marty Makary and Vinay Prasad?
Here is the short NEJM article, which is not behind a paywall and can be reviewed by anyone.

The article starts off well, noting that the US is the only major western nation to recommend yearly COVID boosters for everyone above the age of 6 months, and even has a handy chart for emphasis.

But things go downhill from there. It looks like Makary and Prasad, if indeed they did design or agree to this policy, a big IF, fancy themselves Solomons, and have decided to cut the baby in half and give one half to those demanding COVID vaccines be proven safe before a single person gets another shot, and half to COVID vaccine true believers.
That is not how you run a regulatory agency. The FDA is required to review all the evidence and make a purely scientific determination as to safety, efficacy and necessity, with a nod to cost. They are NEVER supposed to ignore that statutory obligation. Instead it sure looks like they are providing a “glass half full” to each side in a popularity contest.
Snippets follow with my comments:

This says FDA does not know the benefit of COVID boosters, from any year or strain. Duh. In that case, you halt vaccinations until you figure out the benefit. But that is not what these authors suggest.
They could avoid splitting the baby in half by simply balancing safety and efficacy, as is their mandate. Efficacy is a question mark, and safety is awful, and the VAERS data are clearcut on this. So FDA officials would have good reason to NOT recommend boosters to anyone.

Here the authors reveal their ignorance (or contempt?) of vaccine science. Immunogenicity as a replacement for efficacy is a joke. FDA is NOT supposed to accept immunogenicity as a surrogate marker of effectiveness unless it has proof (correlates of immunity) to show that the specific antibodies generated provide actual protection from COVID over the year of expected efficacy. FDA does not have data showing this. Prasad is a cancer doctor and epidemiologist and Makary is a surgeon. Neither one is knowledgeable about vaccine science, obviously. And if FDA staffers who ARE knowledgeable about FDA’s regulations actually wrote the policy and the article, were they deliberately hanging Drs. Makary and Prasad out to dry? To take the fall? To please nobody?
The sentence below in blue was probably not written by either Dr. Makary or Prasad. It references a legal nicety they are unlikely to know: FDA needs to claim it has an efficacy endpoint to legally justify a license, and so the author of this sentence is claiming that since FDA asserts that antibody titers are good enough evidence for efficacy, then FDA has the legal requirement to issue a license.

I am guessing that clever Dr. Prasad came up with a model for epidemiology studies of efficacy that are very satisfying methodologically, but they are disgusting when you see that he is recommending clinical trials in babies and never considers safety. “Risks” for little children? What risks? The risk of a cold or the risk of death? How vague. How cold-hearted.
The rest is addressed directly to the authors.
Dear Authors:
You have not mentioned vaccine safety once in this piece! Are you asleep at the switch? Your job is to provide us safe products. I understand that cancer doctors are accustomed to giving their patients deadly concoctions—but you are not in Kansas anymore, Dr. Prasad. We don’t give vaccines to healthy people that are more dangerous than the disease the “vaccines” are alleged to prevent.
We don’t give vaccines to people who are not healthy (your 100-200 million Americans who will be “eligible” for COVID vaccines under your new policy) if they don’’t provide net benefit—which seems to split the US population in half: the vaccines were recommended for 340 million Americans till now, and going forward will be recommended for half of them.

WTF is regulatory flexibility? Flexibility means the FDA can do whatever it wants, evidence be damned.
So-called “high risk” persons will be given vaccines without evidence, while for healthy people evidence is needed. I believe that flies in the face of the Constitution’s 14th Amendment, “Equal Protection.” It won’t fly.
Your article indicates that safety continues to be ignored by the FDA. That is simply impermissible, since in order to license a drug or vaccine there must be a risk-benefit assessment, which you have failed to either perform or discuss in this piece.
This article is a slap in the face to the American people and to the legal and scientific process of regulation. Those who wrote the policy and the article are unfit to regulate America’s drugs and vaccines. I would venture to guess they were not Drs. Makary and Prasad.
There are only a few explanations for this policy, and none of them are good:
- Ignorance of regulation, rules, and the entirety of the data on COVID vaccine safety and efficacy
- Insubordination by staff, who are trying to throw their new leaders under the bus
- Hubris: choosing to regulate by fiat and clever ideas, lacking a mooring in the existing body of regulatory science and rules
We must have excellent regulation of medical products if the population is to become healthier.
Please be brave and dismiss this policy and start over.
Addendum: yesterday’s NYT said the following:
A spokesman for the agency later said that clinical trials would be required, not just recommended or anticipated.
Vaccine manufacturers should follow participants for at least six months and evaluate whether the effect of the vaccine persists over that time, Dr. Prasad said during the livestream.
Following subjects for six months is designed to miss the period of negative efficacy that begins around 6 months in adults (though sooner in small children) according to the CDC. See below how quickly efficacy becomes negative for the first shots. It may go to negative sooner for the boosters.
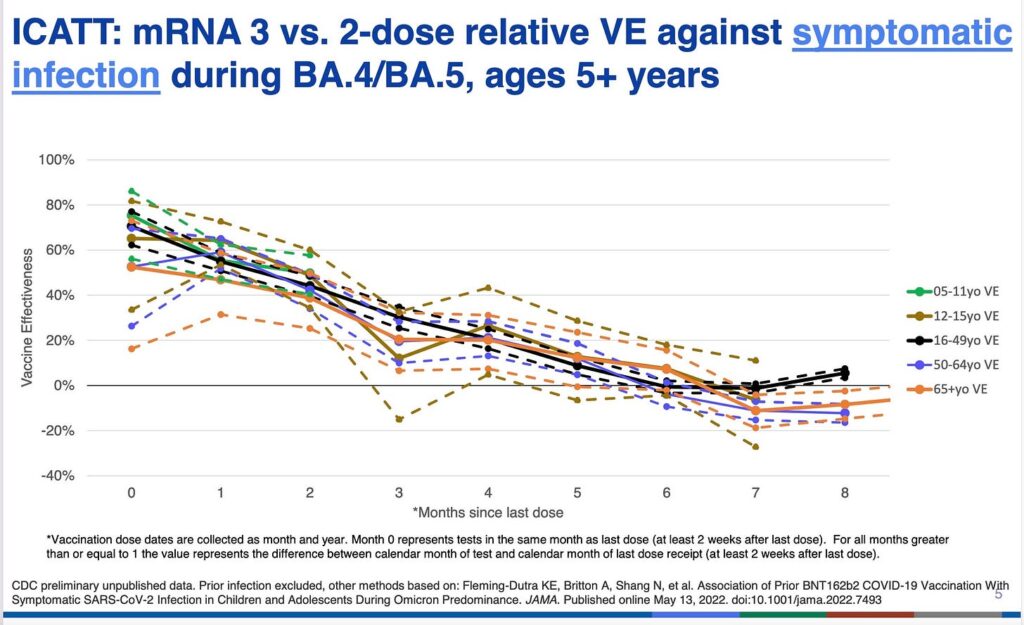
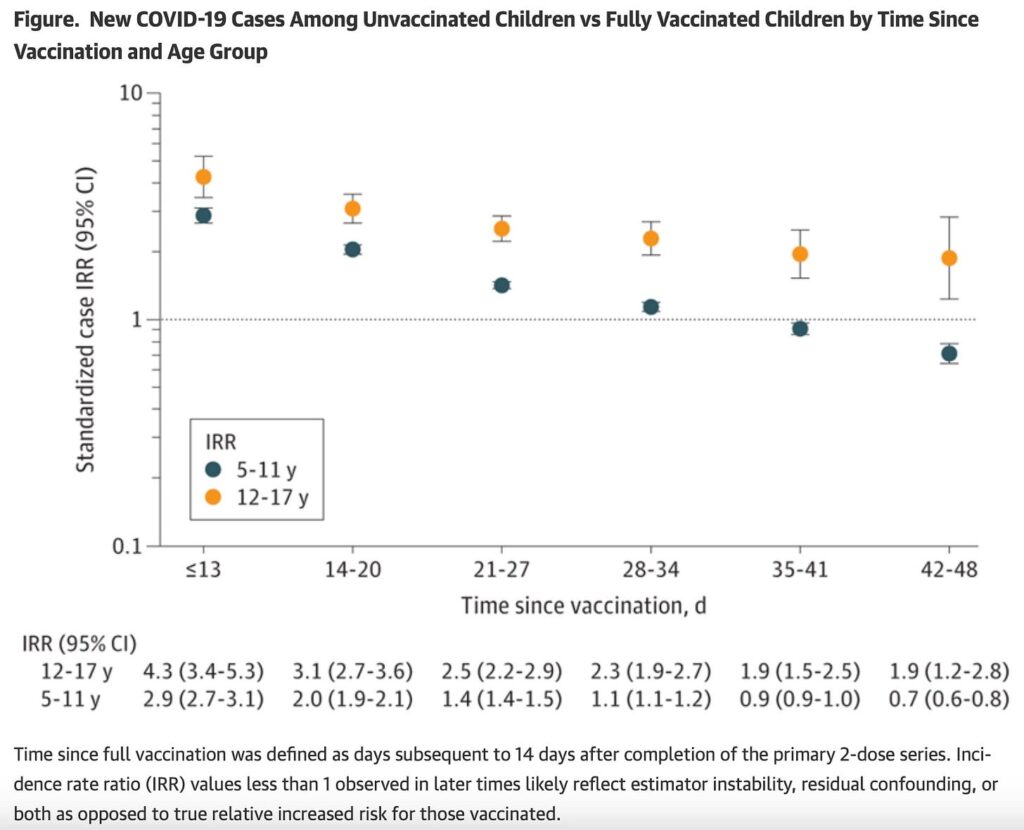
And for children aged 5-11 efficacy dropped off much quicker after the initial shots—before 2 months, in data from NY state reviewed by CDC. Dorabwila was the first author of the JAMA paper.
From the NYT:
In a statement, Pfizer said it is evaluating the details shared Tuesday and is in ongoing discussion with the F.D.A. The company said its Covid vaccines have been given to over a billion people, “generating robust data demonstrating a favorable safety profile. “
Moderna, maker of another widely used Covid vaccine, said it appreciated the F.DA.’s clear guidance and would work with the agency to provide the data to ensure access to its vaccine for Americans.