Pfizer trial of modRNA (the new name, which acknowledges the modified uridine in mRNA vaccines) FLU SHOT published in the NEJM today
Pfizer trial of modRNA (the new name, which acknowledges the modified uridine in mRNA vaccines) FLU SHOT published in the NEJM today
And it has no paywall, so Pfizer paid extra for that benefit; they must be so proud of the poor results
https://www.nejm.org/doi/full/10.1056/NEJMoa2416779?query=TOC
Let me state the conclusions from simply reading the abstract of this paper:
-
Efficacy is claimed to be 35% better than the 2022-23 quadrivalent vaccine against which the experimental mRNA vaccine was tested
-
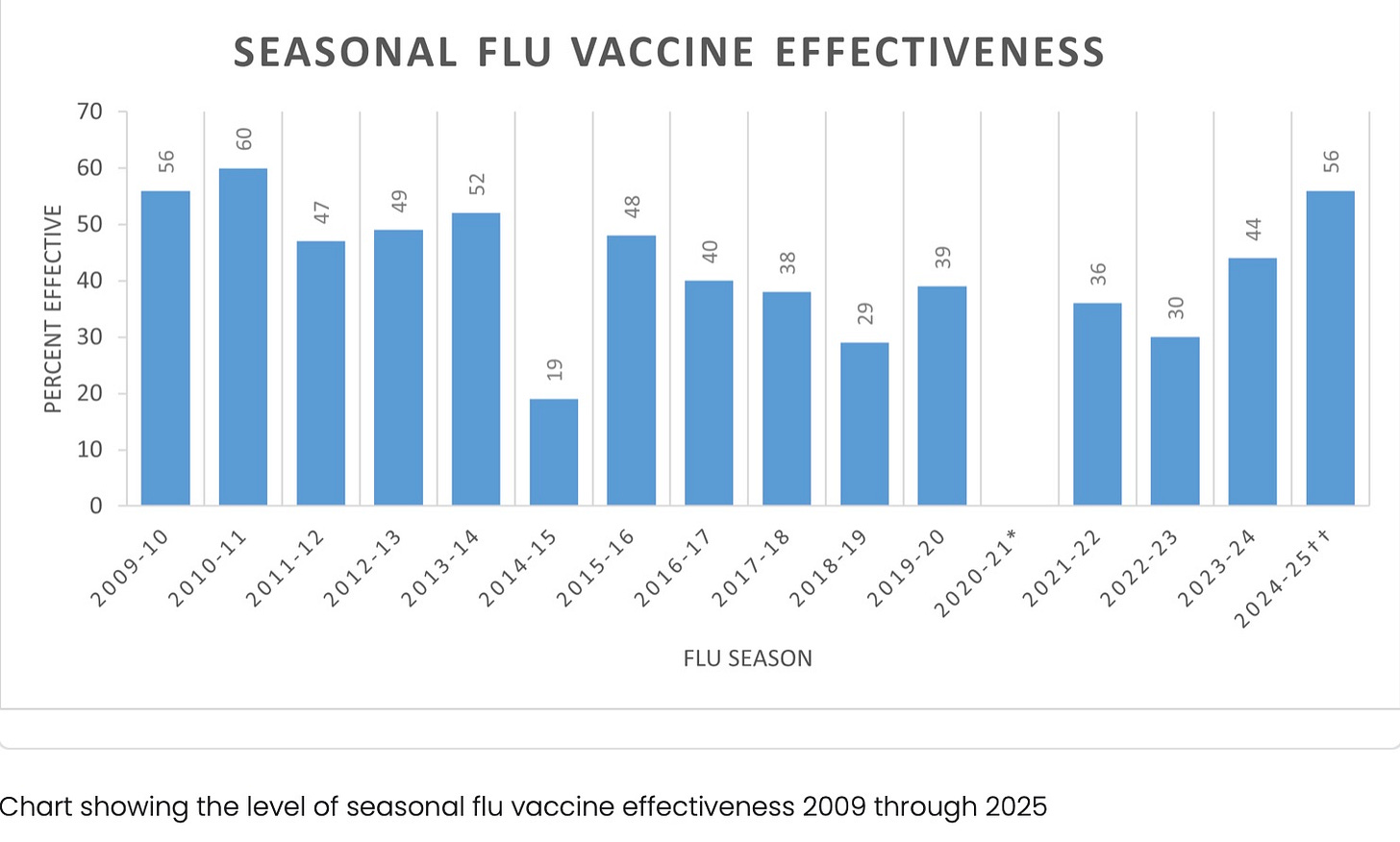
However, in the CDC’s study of effectiveness (from its own network, so impossible for us to verify veracity of its efficacy claim) in 2022-23, for the standard flu vaccines, effectiveness was only 30%.
https://www.cdc.gov/flu-vaccines-work/php/effectiveness-studies/index.html
So if this vaccine was 35% better than 30%, it would have 40.5% efficacy.
-
However, the alleged increase in efficacy must be viewed in light of its worse safety profile, when compared to the comparator flu vaccine used, Fluzone. BTW, the standard Fluzone vaccine contains 25 mcg mercury per adult dose, when used in multi-dose but not single dose vials. The Fluzone label indicates a fairly high rate of systemic reactions associated with this standard (non-RNA) flu shot: https://www.fda.gov/media/170019/download?attachment
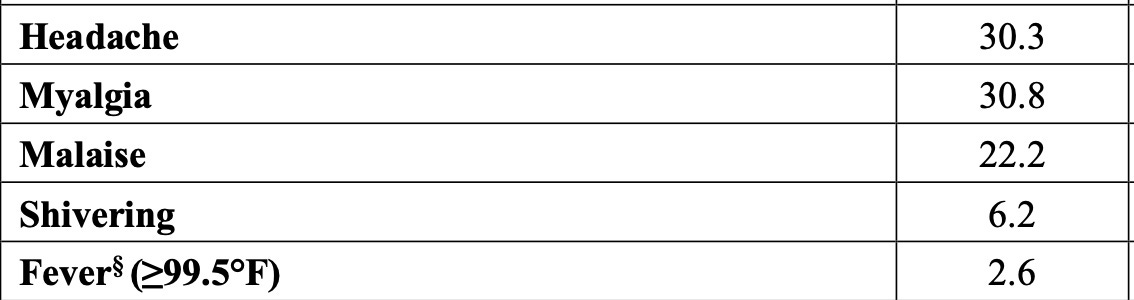
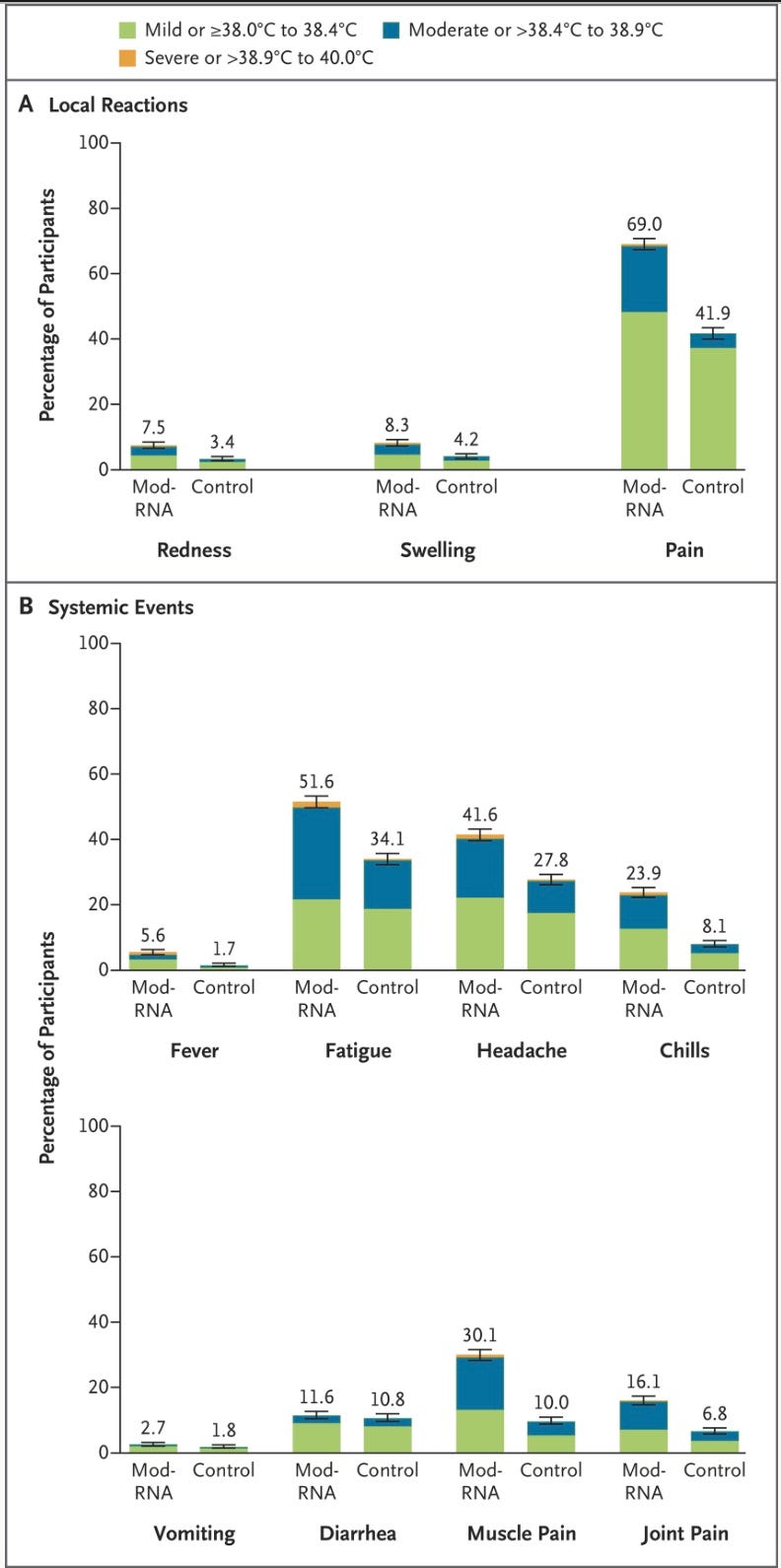
Among 6127 participants in the reactogenicity electronic-diary subgroup, recipients of the modRNA vaccine reported more local reactions and systemic events than control recipients (70.1% vs. 43.1% for local reactions and 65.8% vs. 48.7% for systemic events) (Figure 5 and Table S8).
Below it is apparent that the Pfizer modRNA vaccine causes more adverse reactions of every type compared to the Fluzone, the standard (comparator) flu shot.
-
The vaccine contained antigens for 2 influenza A strains and 2 influenza B strains. The two influenza B antigens did not generate the desired immune response—but they tell us that is okay because there were hardly any influenza B cases in the trial subjects—so don’t worry about the 50% of the vaccine that is not up to snuff.
-
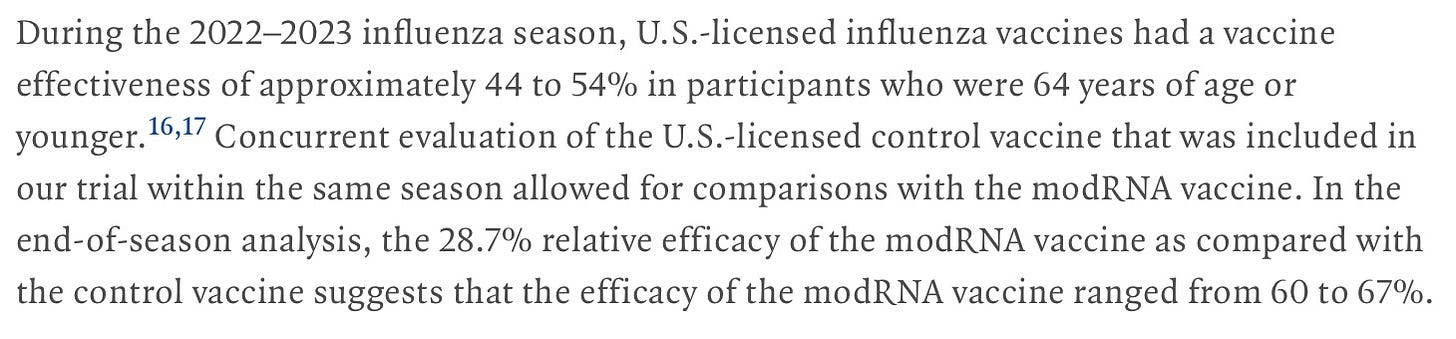
The authors of the study appear to have lied about the efficacy of the comparator vaccine—see what they have claimed, then look at the CDC chart above regarding flu shot efficacy in 2022-23: instead of 30% efficacy, they claim efficacy was 44-54%, and since their modRNA vaccine was 35% better, it had the amazing efficacy for a flu shot of 60-67%.
-
Lisa Jackson, the third author of this paper, appears to have forgotten what she acknowledged in 2008 (see below), which was that it was very controversial whether flu shots worked to prevent flu deaths at all in the elderly. Yet this is the justification for the flu shot program each year, in which CDC invests billions of taxpayer dollars. So why are we giving out these shots at all? What is the real payoff of flu shots? Is it just to get us to line up yearly for a protective shot—to make this part of our culture? To get us ready (soften us up) for the mRNA jabs?
Here is the NEJM abstract:
Abstract
BACKGROUND
Influenza remains a major health burden despite the use of licensed vaccines. Nucleoside-modified messenger RNA (modRNA) influenza vaccines have shown promising immunogenicity against influenza and an acceptable safety profile in a phase 1–2 trial.
METHODS
In this phase 3 trial, we randomly assigned healthy adults between the ages of 18 and 64 years to receive either a quadrivalent modRNA influenza vaccine (modRNA group) or a licensed inactivated quadrivalent influenza vaccine (control group) during the 2022–2023 influenza season in the United States, South Africa, and the Philippines. The primary end point was relative efficacy, defined by the reduction in the percentage of participants with laboratory-confirmed influenza associated with influenza-like illness at least 14 days after vaccination with the modRNA vaccine, as compared with the control vaccine, and analyzed for noninferiority and superiority. Immunogenicity was evaluated by means of a hemagglutination inhibition (HAI) assay. We assessed reactogenicity within 7 days after vaccination, adverse events through 1 month, and serious adverse events through 6 months. We assessed vaccine efficacy, immunogenicity, and safety in the modRNA group.
RESULTS
A total of 18,476 participants underwent randomization: 9225 were assigned to receive the modRNA vaccine and 9251 to receive the control vaccine. The relative efficacy of the modRNA vaccine as compared with the control vaccine against influenza-like illness was 34.5% (95% confidence interval [CI], 7.4 to 53.9) on the basis of 57 cases in the modRNA group and 87 cases in the control group, a finding that met the criteria for both noninferiority and superiority. Cases of influenza-like illness were caused by A/H3N2 and A/H1N1 strains but almost no B strains. The noninferiority of the antibody response on HAI assay was shown for influenza A strains but not for B strains. Primarily mild or moderate reactogenicity was observed in both vaccine groups but was reported more frequently in the modRNA group (overall local reactions, 70.1% vs. 43.1%; overall systemic events, 65.8% vs. 48.7%). Fever occurred in 5.6% of the participants in the modRNA group and in 1.7% of those in the control group. Adverse event profiles were similar in the two groups.
CONCLUSIONS
The modRNA vaccine had statistically superior efficacy over the control vaccine, with greater immune responses to A/H3N2 and A/H1N1 strains, but was associated with more reactogenicity events. (Funded by Pfizer; C4781004 ClinicalTrials.gov number, NCT05540522.)
See also in NEJM Evidence: Human clinical trial of a nucleoside-modified mRNA influenza vaccine