Public Health Gone Awry: Birth Dose of Hepatitis B Vaccine (IOM chimes in on lack of good vaccine data)
3 old posts of mine regarding Hep B and actual vaccine risks and benefits
Sunday, July 5, 2015
Public Health Gone Awry: Birth Dose of Hepatitis B Vaccine (IOM chimes in on lack of good vaccine data)
Hepatitis B is a serious disease. It is highly prevalent in many countries in Asia (nearly 10% of Chinese carried the illness in 1992), but fortunately is of low prevalence in the US. Per CDC, the overall reported US incidence rate for 2013 was 1.0 case per 100,000 population. After adjusting for under-ascertainment and under-reporting, an estimated 19,764 acute hepatitis B cases occurred in the US in 2013.
Hepatitis B is due to a virus transmitted by sex, vertical transmission from mother to newborn, from blood products and iv drug abuse primarily. There is no casual transmission. The graphs below show that disease rates in all age groups were dropping even before hepatitis B vaccine was recommended for children with no risk factors, in late 1991.
Acute hepatitis B cases in children are now extraordinarily low: according to CDC, they have fallen “from 1.2 cases per 100,000 population in 1990 to 0.02 cases per 100,000 population in 2007,” or one new, acute case per 5 million children per year. (The same CDC report notes there were 87 perinatal cases reported from 22 states in 2007, a higher incidence. I am guessing that the perinatal cases were detected via maternal screening, while the prior estimate was derived from new clinical cases in older children.)
The reduction in disease incidence is fantastic. CDC’s recommendation that all pregnant women be screened for hepatitis B in 1988, and that they and their newborns be treated, is likely the major reason for the decline in pediatric and young adult cases.
But CDC didn’t stop there. CDC made stepwise recommendations for hepatitis B vaccinations for all children (who were by age at lowest risk of hepatitis B) during the 1990s, and specified a dose at birth for all newborns in 2002, even though the risk to newborns with hepatitis B negative mothers was close to zero even then. (A well-publicized controversy in France over hepatitis B vaccine-induced multiple sclerosis in the mid 1990s had affected vaccination acceptance here. The multiple sclerosis issue was never fully resolved, while the birth dose of hepatitis B vaccine may have contributed to greater vaccine uptake in the US, according to CDC.)
What do other countries with low prevalence of the disease do with respect to vaccinating newborns, a strategy that only helps those from Hepatitis B-infected families? Data on vaccine policy for all countries in Europe can be found on the ECDC website.
Only 6 of europe’s 31 countries recommend a dose of hepatitis B vaccine for newborns whose mothers are Hepatitis-B negative: Bulgaria, Lithuania, Poland, Portugal, Romania and Spain.
Incidence* of acute hepatitis B, by age group and year — United States, 1990–2007
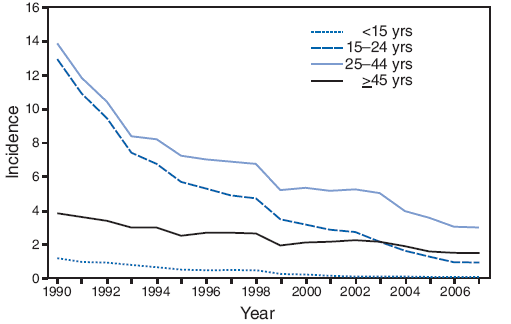
* Per 100,000 population.
Incidence* of acute hepatitis B, by sex and year — United States, 1990–2007
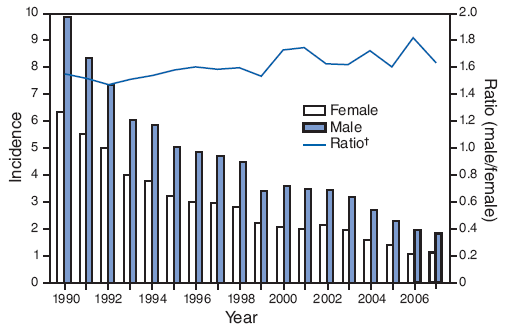
* Per 100,000 population.
† The bars indicate the rate per 100,000 (left y-axis) by sex; the line is the ratio (right y-axis) of the incidence rate among males compared with that among females.
But here’s the thing:
Public health decisions must balance risk and benefit to the entire population when determining policy. In the case of vaccinations, it can be devilishly difficult to ascertain vaccine causality: the true rates, types and severity of adverse reactions remain questionable, since they may be delayed by weeks, months or even years following a vaccination. (Local reactions, rarely severe, such as redness, swelling, or tenderness at the injection site are easy to assign to a vaccine, while systemic reactions are not.)
When a disease is common in the population, in order to reduce its incidence via vaccinations, one can accept a certain degree of (estimated) adverse events due to vaccinations.
When a disease is rare, but you are still vaccinating the same number of people, the number of vaccine-induced adverse events does not drop–but your population benefit is reduced, since you are preventing fewer cases. Given the reduced benefit, the adverse reactions may no longer be counterbalanced by the population benefit. But have CDC’s public health professionals reevaluated their hepatitis B vaccine policy in light of current disease rates?
There is a big, big problem at the heart of vaccination policies: there simply are no reliable adverse reaction data. In the specific case of hepatitis B vaccine, a newborn gets vaccinated on the first day of its life, and since the parents have no prior experience with that baby, it is especially difficult to identify a systemic adverse reaction to the initial dose of vaccine.
One “non-systematic” review reported: “After reviewing the literature, we observed that complications seen after Hepatitis B vaccination are sudden infant death syndrome, multiple sclerosis, chronic fatigue syndrome, idiopathic thrombocytopenic purpura, vasculititis, optic neuritis, anaphylaxis, systemic lupus erythematosus, lichen planus and neuro-muscular disorder.” Observed. Not measured. Who knows what it means?
The label for Glaxo’s Engerix-B vaccine is vague regarding causality and severity of adverse events. A WHO information sheet on Hepatitis B adverse reactions is equally troubling, as the literature it cites is full of contradictions. Hand-waving by WHO to dismiss unwanted findings is unconvincing, when a vaccination policy appropriate for reducing hepatitis B transmission to newborns in low resource areas of the world (areas without assured screening during pregnancy) has been imposed in the US on our high resource, highly screened population of mothers and infants.
I know that what I am writing seems difficult to believe. How could vaccines be licensed by FDA without good adverse event data? But the fact is that when they are licensed, all the data FDA evaluates have been generated by the manufacturer. Later, it is usually not beneficial to the manufacturer to actively seek evidence of harm.
The US government requests advice from the Institute of Medicine (founded in 1970 and part of the National Academy of Sciences, chartered by Congress in 1863) regarding issues of public health.
In 2012, an Institute of Medicine which had been asked to investigate the adverse event literature for Hepatitis B and other vaccines, issued this report:
Adverse Effects of Vaccines: Evidence and Causality
Editors
Committee to Review Adverse Effects of Vaccines; Institute of Medicine; Stratton K, Ford A, Rusch E, Clayton EW, editors. Washington (DC): National Academies Press (US); 2012.
The report’s conclusions state:
The committee acknowledges that some readers may have concerns about two aspects of the report. First, the committee does not make conclusions about how frequently vaccine adverse events occur. Secondly, the committee concluded, for most analyses, that the evidence is inadequate to accept or reject a causal relationship and some readers might interpret the committee’s language in different and inaccurate ways. The committee offers concluding comments to address these two issues.
This report is not intended to answer the question “Are vaccines safe?”. The committee was not charged with answering that question. Other bodies make that determination and contribute to ongoing safety monitoring, including governmental agencies, care providers, and industry, as they determine the benefits and risks of marketing a product. At all levels, policy determining vaccine use requires a balancing of risks and benefits. As described in Chapter 1 and the Preface, that is outside the bounds of this committee’s assignment. It should also be noted that where the committee has found evidence of a causal relationship, it does not make conclusions about the rate or incidence of these adverse effects.
Determining the rate of specific adverse events following immunization, in the general population or a subset thereof, is challenging. It would be possible, for example, to estimate a rate of the occurrence of a specific adverse effect in a vaccinated population or susceptible subgroup of interest. This could be done using a summary relative risk or absolute risk difference (e.g., estimated from a set of consistent reports reviewed by the committee) if there were large population-based studies of the occurrence of the adverse event in unvaccinated individuals (e.g., in the general population or susceptible subgroups of interest) who do not substantially differ from those vaccinated on any known, important confounders (e.g., age and exposure to other vaccinations or other agents or factors known to cause the adverse event). None of these preconditions is fully met for the adverse events reviewed in this report.
The committee also notes here that large epidemiologic studies that report no cases of the adverse event of interest in vaccinated study participants, if included in our analyses, raise particular concerns. If at least some cases of the adverse event occurred in a study’s unvaccinated comparison population, an upper limit of the 95% confidence interval (CI) for the study’s relative risk or absolute risk difference could be estimated, but one would be unable to rule out a possibly increased risk unless the vaccine was significantly protective against that particular adverse effect. Also, including such studies may have exacerbated problems with detection biases unless precautions were taken to ensure equal surveillance for the adverse event in the unvaccinated and vaccinated populations being compared…
So there it is. In the absence of good data, it is hard to make good public health decisions–but they get made nonetheless, and the decision-making is population-based, independent of any individual’s unique risk-benefit profile.
But for an individual, you can make an assessment that includes your risk factors for the disease, the vaccine efficacy (very good for Hepatitis B vaccine, although duration of protection is uncertain) and see how that balances with the (unknown) degree of risk from the vaccination. If you are at more than infinitesimal risk for the disease, include a factor for the considerable health risks inherent in the disease, were you to develop it in the absence of vaccination. Caveat emptor.
Sunday, April 26, 2015
Why Would You Vaccinate a Newborn for Hepatitis B?My maternal grandfather died in 1968 of what was almost certainly a fulminant Hepatitis B infection (causing rapid liver failure, occurring in just 1% of acute cases). He probably acquired it working part-time in a deli, slicing meat. In med school a few years later, I learned that butchers had increased risk. You get Hepatitis B from blood (needle sharing, needlesticks) and sex. And very rarely from activities where blood from an infected person can contaminate a fresh cut. A newborn can catch it from its mother, too.When I was an intern I treated a young prisoner who had been an intravenous drug abuser and had both bacterial endocarditis with an acutely flailing mitral valve and active Hepatitis B. We learned this when a nurse got a needlestick. His terrible heart failure led to bloody pulmonary discharge sprayed on us all. We were lucky to keep him alive, struggling over him all night, until he had surgery for a valve replacement the next day. His treatment team was offered the new Hepatitis B vaccine, made from pooled blood (no longer used) and Hepatitis B immune globulin (also made from pooled blood, still used occasionally). I accepted both. After all, I might have a genetic predisposition to fulminant Hepatitis B. I even paid cash for the vaccine. I weighed the benefit and risk, and the vaccine won.Now it is 2015 and I am expecting twin grandchildren, a boy and a girl, in July. If a bill passes the Vermont House (it already passed the Senate), they will both receive a dose of Hepatitis B vaccine on the day of birth… if they hope to attend day care, or any public or private school in Vermont. Since we do not expect them to use a meat slicer, have sex or play with dirty needles, what could the reason for vaccination at this age possibly be? All moms are screened (99% screening rate in New Hampshire for expectant moms) so an infected Mom is unlikely to slip through the cracks. An infected mom and her newborn need not only the vaccine, but also immune globulin, and possibly an antiviral drug. So vaccinating all babies does not provide adequate treatment for those who really need it.When newer Hepatitis B vaccines became available, they were recommended only for those at high risk. But many high risk individuals did not choose to be vaccinated. So the decision was made to instead vaccinate infants. Infants may be at infinitesimal risk, but they will eventually grow to an age where their risk increases. They are a captive audience. Infants can’t say “no” to a vaccine, like their parents can, and do. It makes sense, I guess, if your goal is to reduce numbers of cases using the most easily-imposed route. It makes some sense at the population level. It makes sense if there are almost no side effects from the vaccine.But what if there are side effects? Babies cannot tell you if they are experiencing a side effect. What if the birth dose contributes to later childhood neurologic problems in those who are susceptible?When your new baby is vaccinated on the day of birth, you don’t know what that child might have been like, without being vaccinated. You cannot compare “before” and “after.” You cannot easily determine what is a side effect from that very first dose of vaccine. A colleague of mine, who had access to vast amounts of unpublished data on this vaccine as an expert for the French government, wrote about the difficulty of acknowledging and understanding the vaccine’s adverse reactions, when so much important information has never been published.Below, CDC maps out the chronology of its Hepatitis B vaccine program’s 20 year mission creep, from recommendations for those who really needed it, to those who clearly do not:Even before CDC recommended that all babies be vaccinated, in 1990, there were less than 2 new Hepatitis B cases per 100,000 per year, in US children under age 15. From CDC:We are vaccinating 4 million newborns yearly to prevent a disease that occurs in less than 25 children aged 0-12 months each year. In fact, the vast majority of vaccinated children are at zero risk of the disease. According to CDC, among all US children aged 0-19 years, in 2002 there were only 3 new cases per million children annually. But there may be many fewer cases even than this number. According to medical reference UpToDate by 2007, the incidence of acute hepatitis B in children less than 15 years old had fallen to 1 in 5 million per year. Furthermore, UpToDate tells us, “The majority of [US] children with chronic HBV infections are immigrants, have immigrant parents, or became exposed through other household contacts.” And the majority of new cases in childhood are in children not born in the US.The public health goal, presumably, is to have children immune by the time they becomes sexually active or at risk from needles. Yet one CDC study showed that half the children vaccinated starting at birth, with 3 doses, lost immunity after 15 years. A Cochrane meta-analysis found that “the prevalence of anti-HBs seroconversion by rapid vaccination (0, 1, and 2 months) was significantly lower than that by standard vaccination (0, 1, and 6 months). If giving the third Hep B vaccine dose later results in better immunity, it is perhaps likely that giving the first 2 shots later would improve immunity. Two studies (here and here) showed non-detectable Hep B antibody levels 6-10 years later in nearly 50% of children who were vaccinated starting at birth, though most did show evidence of immune memory when given a booster dose. But it would be preferable to have persisting high antibody levels, in addition to immune lymphocytes, if vaccinated children were later exposed to the hepatitis B virus. Likely this would be better achieved by starting vaccinations in low-risk children at an older age, or not at all, as many other countries do.Vaccinating at birth is illogical for low-risk babies, if what you seek is to retain immunity into adolescence and adulthood. For that, they should be vaccinated later.UPDATE: Is giving dose of hepatitis B vaccine on the day a child is born, to children whose mothers are negative and have no risk factors, a standard procedure in other countries? Of course not. The European Center for Disease Prevention and Control lists the vaccine schedule for each of the countries in Europe. Only 5 of the 31 European countries (Estonia, Lithuania, Poland, Portugal and Romania) recommend a hepatitis B vaccination for even low-risk babies at birth.Absent an infected mother (less than 0.5% of new moms), it makes no scientific sense to vaccinate every baby on the first day of life. And because babies cannot spread this virus to each other (there is no oral-fecal spread for this type of hepatitis virus) you cannot justify vaccinations of those at extremely low risk of the disease to protect other children.UPDATE: The only possible legal and ethical justification for a vaccine mandate is to protect the public: in this case, other children. Why else would laws be enacted to prevent uninfected but unvaccinated children from attending school? (Remember: infected children are not prevented from attending school!) Since the hepatitis B vaccine fails this standard, it seems the primary protection the Hepatitis B vaccine mandate provides is to the vaccine manufacturers’ profits.Saturday, March 2, 2019Childhood Vaccines that are Unjustifiably Mandated
Meryl Nass, MD
March 3, 2019
Sixteen different vaccines are recommended for all American children by the CDC. The schedule for delivering multiple doses of each can be found here.
-
Three of these are used for diseases have been wiped out, and there is less than a one in 50 million chance that a child in the United States could be exposed to these illnesses.
-
These illnesses are polio, rubella, and diphtheria.
-
Two additional vaccines are for illnesses that are not transmitted between children: tetanus and Hepatitis B. They cannot be spread by casual contact.
-
The Men A vaccine program requires that one million children be vaccinated to prevent one case of disease.
-
Yet most states mandate that children receive these vaccines in order to attend school. The justification to deny an education to children who do not receive these six vaccines–the claim that they would be endangering other children who may be susceptible to the diseases–is false. Then why are they required?
-
And what is the push to give all the adolescents gardasil? It is required to attend school in Rhode Island, Virginia and DC. Governor Perry tried to add Gardasil by fiat to the Texas childhood schedule but the Dems stopped that, and his Merck connections were exposed. Do kids really spread HPV in school? Florida is trying to add it this season. $180/dose, 3 dose series, worldwide it is a 2 billion a year blockbuster. Yet it may cause ovarian failure, POTS, neurologic disorders and miscarriages, but that is totally worth it, as you get to skip a couple of pap smears here and there, right?
-
NO! You still have to get your PAP smears despite your vaccinations, while no one knows how effective the vaccine actually is at preventing cancers.Remember, there are over 100 strains of HPV, but each vaccine only contains antigens against 9 or fewer of them. So you still need condoms, as well as PAP smears.
Polio: The last polio case in the United States due to “wild type” or natural polio was in 1979. Since then, several cases were brought into the US from outside. The last time this happened was in 1993, according to the CDC. In the past 20 years, the handful of polio cases that occurred in the US have been due to infections from viruses derived from live polio vaccines.
For this reason, American children have stopped receiving live polio vaccines and now receive a “killed” or inactivated polio vaccine. But if they are not leaving the US, the likelihood of being exposed to polio is virtually nil. Why must they have four doses of polio vaccine, and boosters if they travel to a country where polio still occurs? Wild-type, natural polio only occurred in only 3 countries in 2016-7, but vaccine strain-derived polio was identified in 15 countries, according to the WHO.
Rubella: Seven cases were reported in the US in 2017. According to the CDC, “Today, less than 10 people in the United States are reported as having rubella each year. Since 2012, all rubella cases had evidence that they were infected when they were living or traveling outside the United States.”
Diphtheria: This is the third disease that has been wiped out in the US. “In the past decade, there were less than five cases of diphtheria in the United States reported to CDC.”
Yet, U.S. children receive six doses of diphtheria vaccine by the age of twelve, 5 from the DTaP and 1 from Tdap vaccine [diphtheria, tetanus and pertussis vaccines].
Hepatitis B: This is a disease that essentially never occurs in young children, unless their parents have transmissible hepatitis B. There were 31 new cases reported in babies under age 2 in 2017 in the US. CDC lists the sources of infection for hepatitis B, which involve sexual activity or blood to blood contact, most commonly from the use of dirty needles:
“Hepatitis B is spread when blood, semen, or other body fluid infected with the Hepatitis B virus enters the body of a person who is not infected. People can become infected with the virus through:
· Birth (a baby whose mother is infected can be infected at or after birth)
· Sharing items such as razors or toothbrushes with an infected person
· Contact with the blood or open sores of an infected person
· Sex with an infected partner
· Sharing needles, syringes, or other drug-injection equipment
· Exposure to blood from needlesticks or other sharp instruments”
Less than 1% of US parents are contagious for hepatitis B, and pregnant women receiving prenatal care are universally tested for hepatitis B. Yet all newborns in the United States are recommended to receive hepatitis B vaccine on the first day of life, regardless of whether their parents are infected or contagious.
· There is simply no reason to give a newborn baby any vaccine on the first day of life, often minutes after birth, and before you know very much about your child. Why subject an unknown and extremely immature immune system to unnecessary toxic substances, which include, in addition to Hepatitis B surface antigen, yeast proteins, aluminum and formaldehyde? There is no medical or public health reason to give newborn babies who are at no risk of infection a vaccine on the first day of life.
· Unless the reason is that any adverse reactions to the Hepatitis B vaccine will most likely be labeled as having been pre-existing at birth.
· To inject a newborn child with substances that cannot help, but certainly may harm them, is public health in reverse.
Tetanus: While tetanus is a very serious neurologic disease caused by bacteria growing in a contaminated wound, it never spreads from person to person. There are about 30 cases of tetanus yearly in the US. Vaccination for tetanus is usually a good idea. However, the plain, single tetanus vaccine (Tetanus Toxoid Adsorbed by Sanofi) was discontinued last year in the US. Tetanus vaccine is now only available coupled to diphtheria vaccine, or to both diphtheria and pertussis vaccines. Why?
Neisseria meningitidis: This is a type of bacteria with at least 12 different serotypes, of which six are known to cause epidemics. Neisseria meningitidis can cause serious cases of meningitis or sepsis. Surprisingly, these bacteria live in the nose or throat of from 1-25% of the population, but the vast majority of people who harbor the bacteria never get sick.
In the US, there is one meningococcal vaccine for 4 serotypes (A,C,W,Y) and a second vaccine for the B serotype. These vaccines are referred to as Men A and Men B. Only the Men A (A,C,W,Y) vaccine is routinely recommended for children aged 12 and 16 in the US. The Men B vaccine may be used if desired. Immunity is not long-lasting from either vaccine.
Over time, long before there was a childhood meningitis vaccine available, Neisseria meningitis infections became rare in the US, for reasons that are completely unknown. In 2017, there were only 107 reported cases of meningitis ACWY in all age groups, in the entire United States–of which only eight cases occurred in the target group of 11 through 23 year olds that might have been prevented by the vaccine. CDC recommends that 8 million children yearly receive this vaccine; one would need to vaccinate a million children to prevent one case. That is, if the vaccine is highly effective. But because there are so few cases, how effective it is in the US population remains unknown.
The Men A vaccine only contains 4 of serotypes, whereas there are at least 12 serotypes of Neisseria meningitis. Additional vaccines could be recommended in future to cover additional serotypes. Rates of illness from the other serotypes have dropped dramatically as well, even though we do not vaccinate against them.
Vaccination does not prevent carriage of meningococcal bacteria in the nose and throat. In fact, several studies have shown that children vaccinated against meningitis have a higher rate of bacterial carriage (in nose and throat) than non-vaccinated children.
While it is uncertain how useful the Men ACWY vaccine is for children, it is clear that it puts some at risk of significant harm. The Menactra brand of ACWY vaccine contains diphtheria toxoid (vaccine) and formaldehyde. The Menactra label notes:
“In adolescents 11 through 18 years of age and adults 18 years through 55 years of age, SAEs (serious adverse events) occurred at a rate of 1.0% following Menactra and at a rate of 1.3% following Menomune – A/C/Y/W-135. In adolescents and adults, SAEs occurred at a rate of 1.3% following booster vaccination with Menactra…”
This is what you need to know: a serious adverse event is really quite serious. FDA has given it a specific definition: it involves a death, a life-threatening event, a permanent disability, a hospitalization or prolongation of an existing hospitalization. This is what you can expect in 1% of the children who receive this vaccine. Why are we doing this?