I did not make clear how bad the risk management plan (RMP) was for the H5N8 Sequirus bird flu “vaccine” so let me lay it out with the entire plan before you
Apologies. It was late last night. This is truly terrible. And the EMA transposed its RMP for Sequirus' H5N1 onto the H5N8 vaccine
Here you can clearly see that the page is about the H5N8 vaccine but much of the required material is from the H5N1 vaccine, which no one even bothered to adapt.
From the page above is the link to this page, prepared for the H5N1 vaccine, which I show you in full below and will discuss:
Above the paper states that “potential risks…need further evaluation” and that missing information “needs to be collected.” Below the paper states “how more information will be obtained”
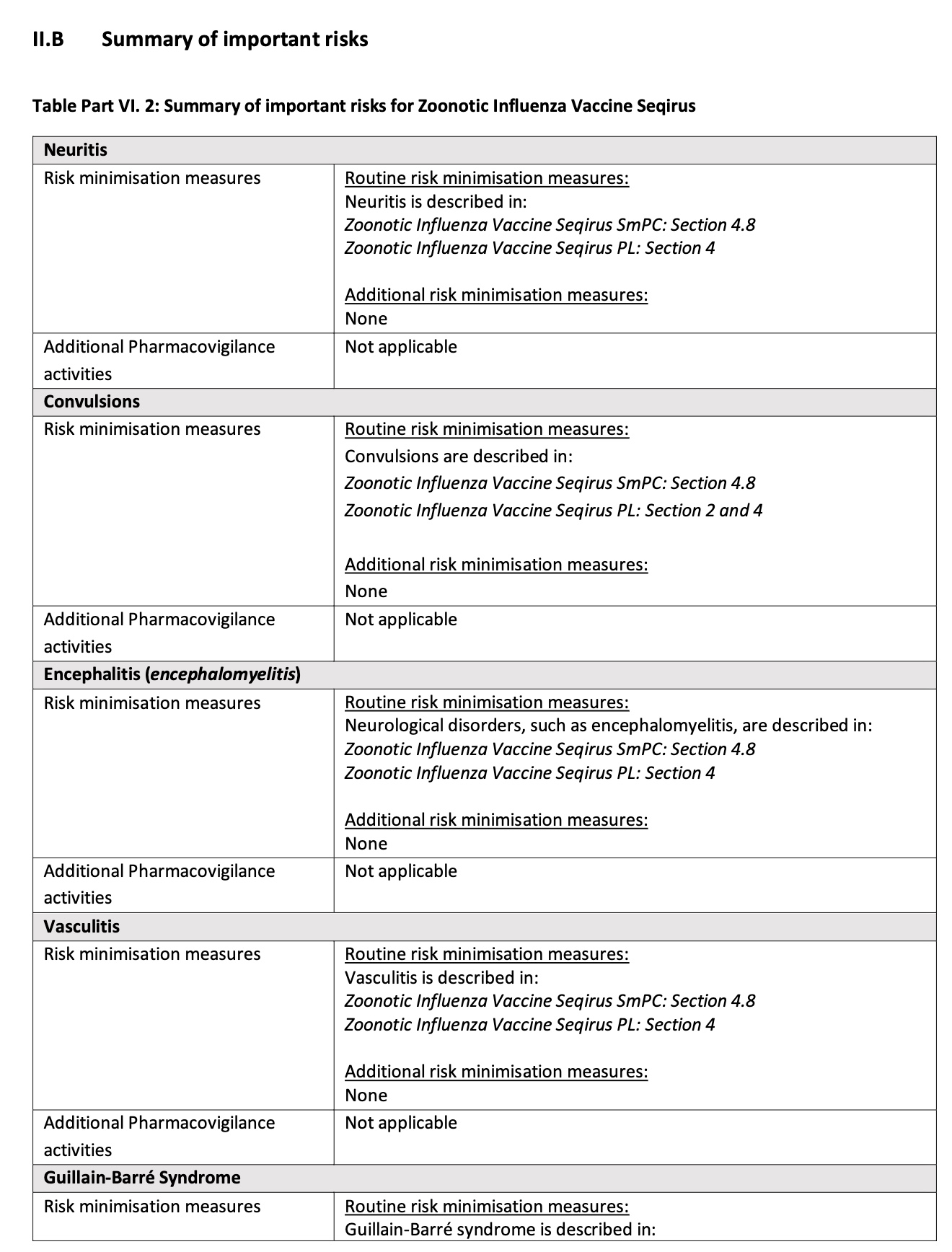
Now we come to the good part where the regulators and manufacturer show that their idea of risk management is only to protect themselves.
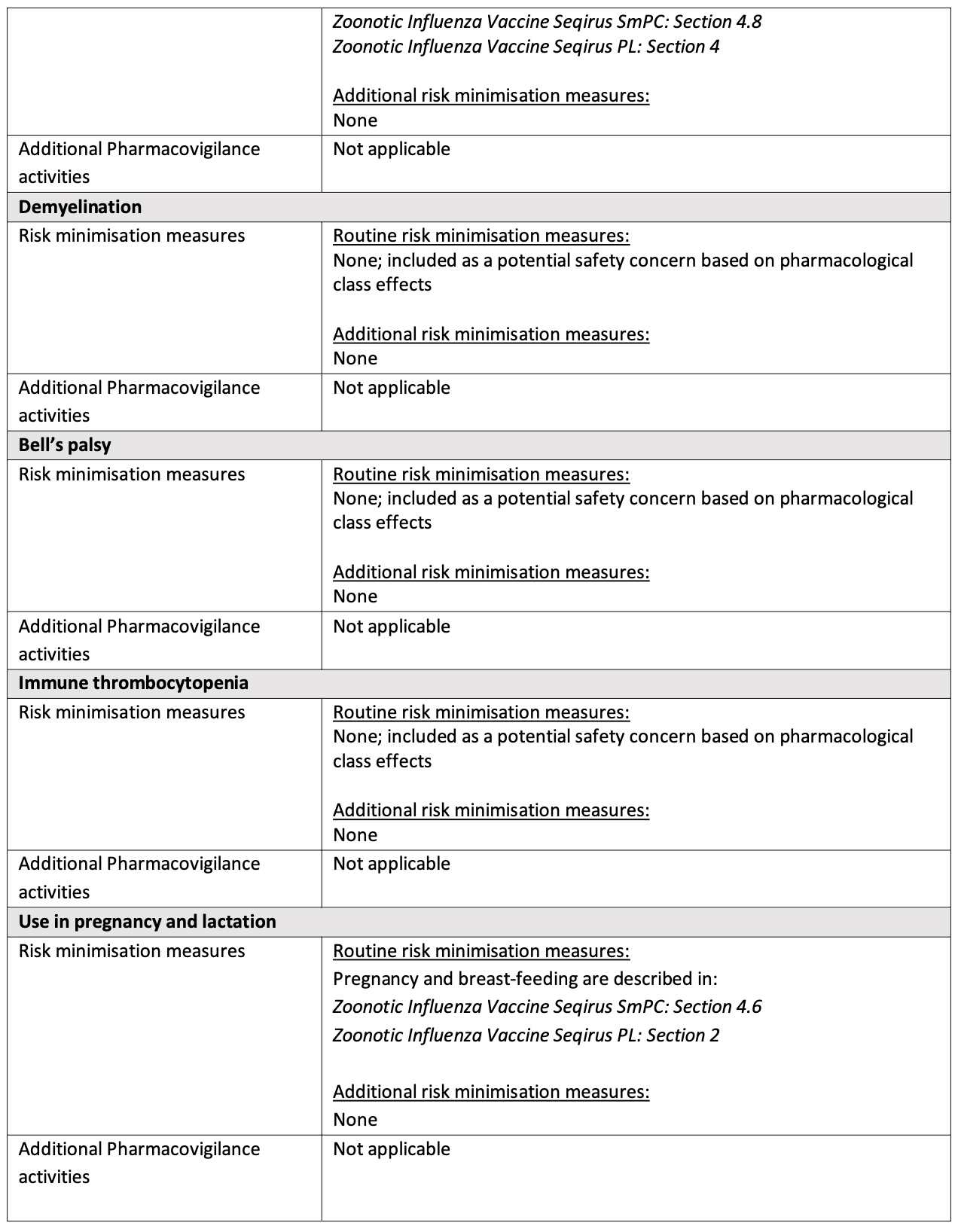
They list each potential serious risk and how it will be minimized. In each case, the risk is minimized by describing it in the package insert.
-
In most cases, no additional risk minimization measures are included.
-
In other cases (like demyelination) they don’t even mention the risk in the package insert
-
Furthermore, pharmacovigilance activities are efforts to collect and analyze data regarding safety. In each case, they are “Not applicable” — meaning there will be no effort to evaluate the risk using data, and there may even be no collection of safety data.
The manufacturer (below) is not being asked to collect any safety data by the regulatory authorities.