FDA’s vaccine advisory committee (VRBPAC) votes to approve Pfizer’s RSV drug in pregnant women with a 10:4 split. 4 new temp members appeared yesterday and all voted to approve.
A sad day for babies and mums
Here is the video of the meeting and my live blog
_________________
Where is the urgency that justifies jabbing pregnant women to prevent RSV infections in their babies (for about 6 months of protection, and at most 12 months)?
Is there a reason to trust Pfizer’s data on its RSV vaccine, when we could not trust its COVID vaccine data?
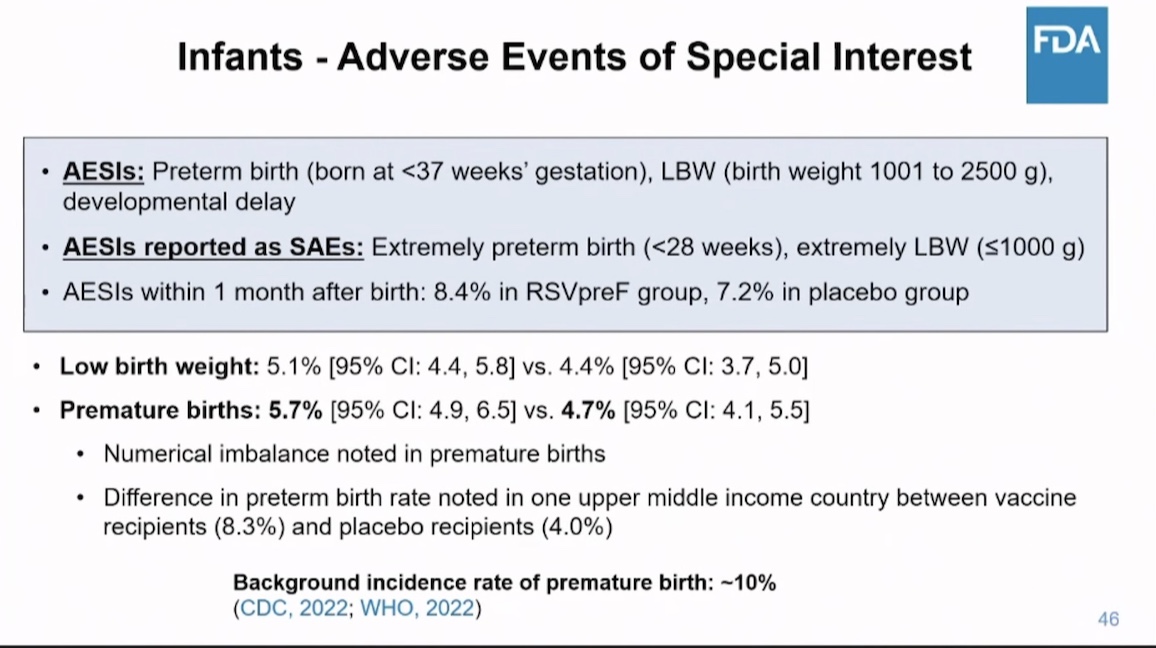
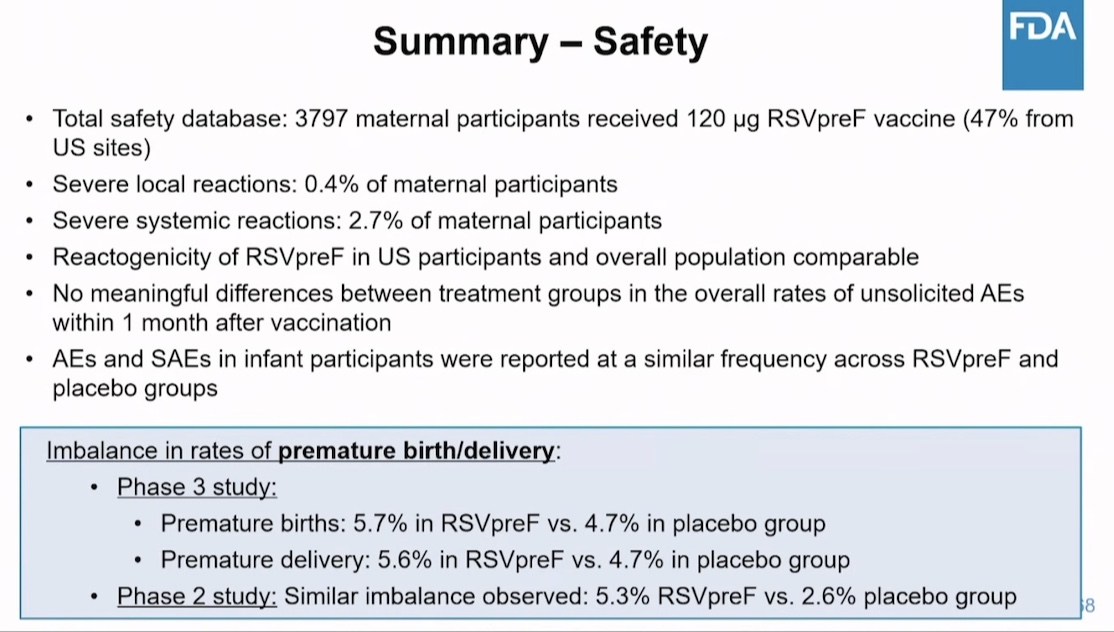
Pfizer found 15-20% more preterm births and low birth weight babies from the mothers who were jabbed, compared to mothers who received placebo.
GSK was developing an almost identical vaccine using the same protein in a different conformation, and shut down development due to its clinical trial results.
How many American babies die from RSV, which affects them mainly between ages 1 and 6 months, causes mostly colds, but causes bronchiolitis, pneumonia and similar lower respiratory infections that can require hospitalization.
As a consequence of the 21st Century Cures Act of 2016, all vaccines recommended by CDC for pregnant women have all manufacturer liability waived, and are placed in the national vaccine injury compensation program. This improves profitability and may result in mandates.
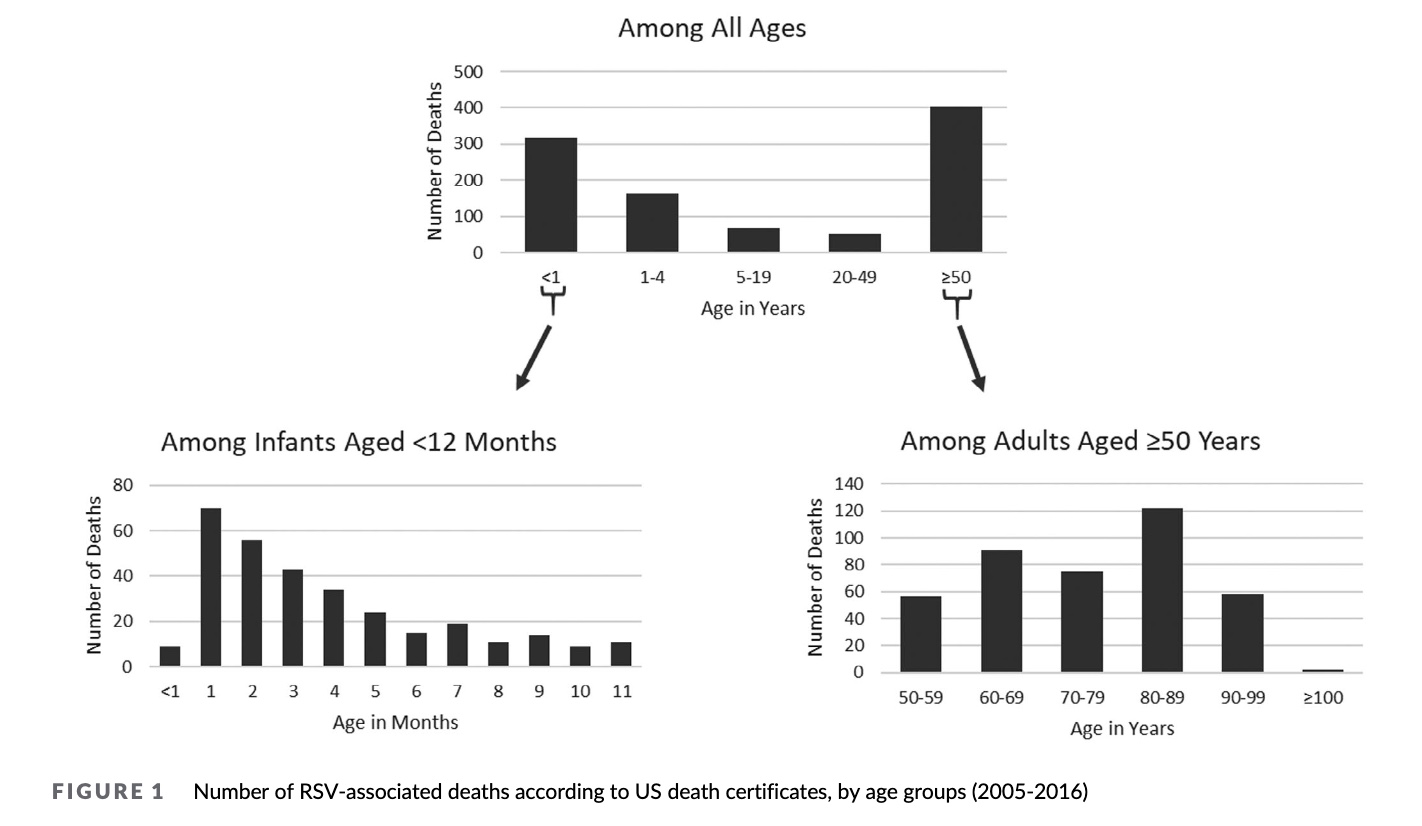
So, how many children die from RSV? The CDC, which collects all death certificates, told us in the following paper it published in 2021. Over a 12 year period, it could only find 300 death certificates in children under 1 year of age who died from RSV, or an average of 25 per year. They think there must be more. So while CDC guesstimates (elsewhere) 300-500 deaths/year, they have nothing to back themselves up, since death certificate data is the gold standard.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8562311/pdf/HSR2-4-e428.pdf
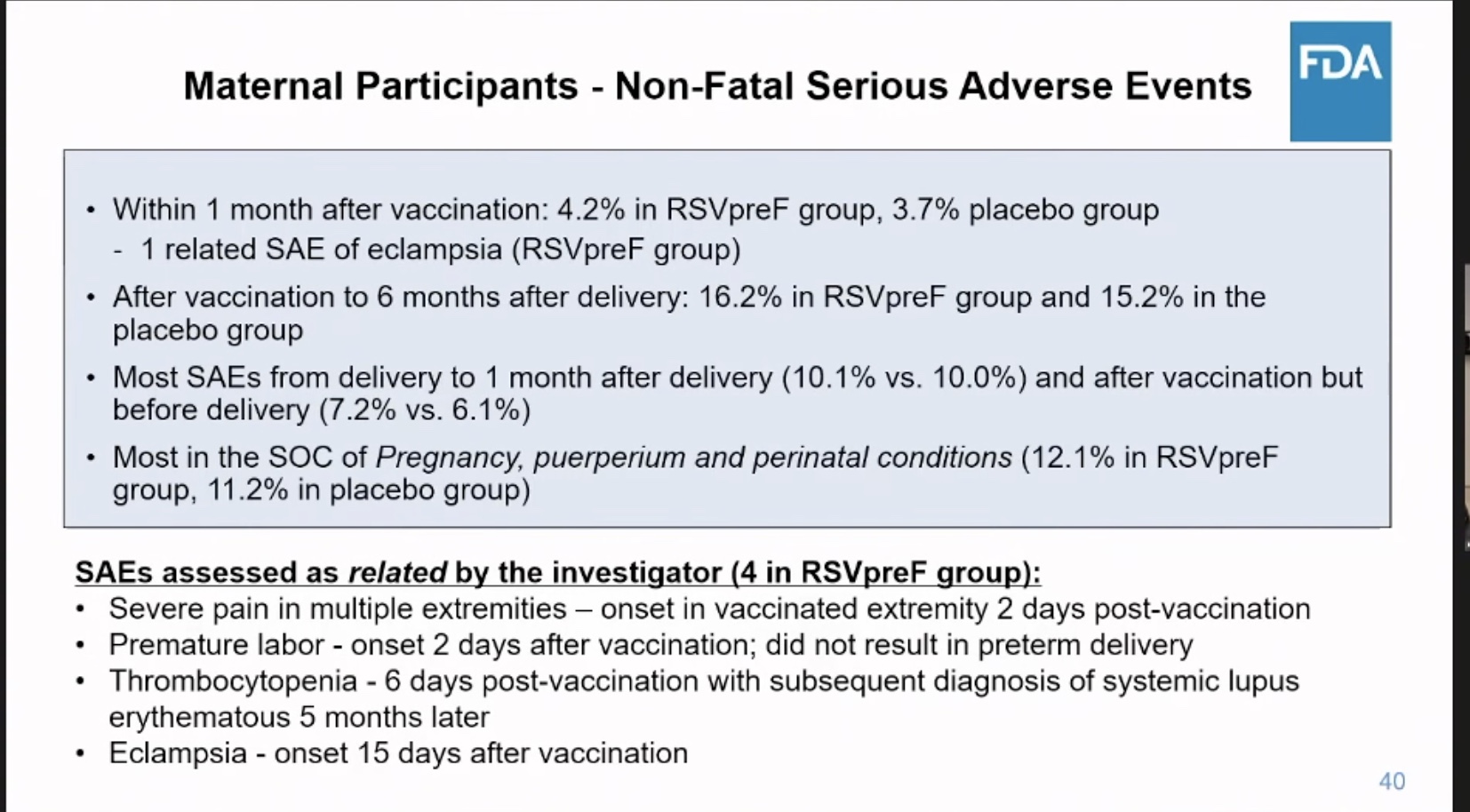
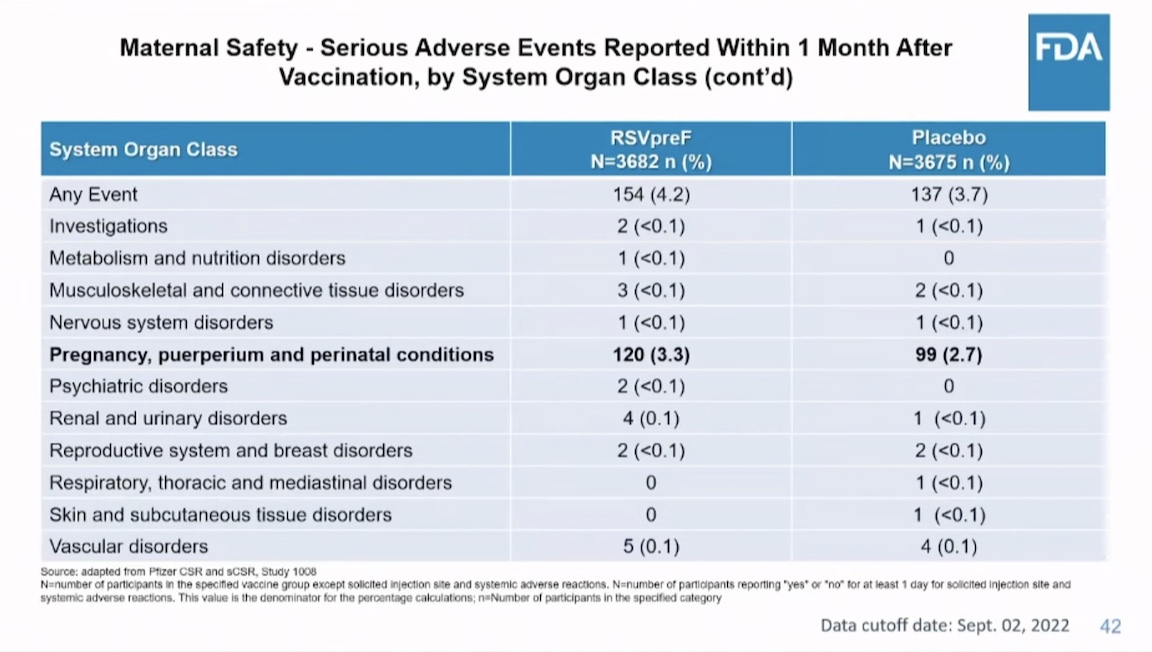
Was the vaccine safe in mothers? Not really. They had more pre-eclampsia and eclampsia and more serious adverse events of other types.
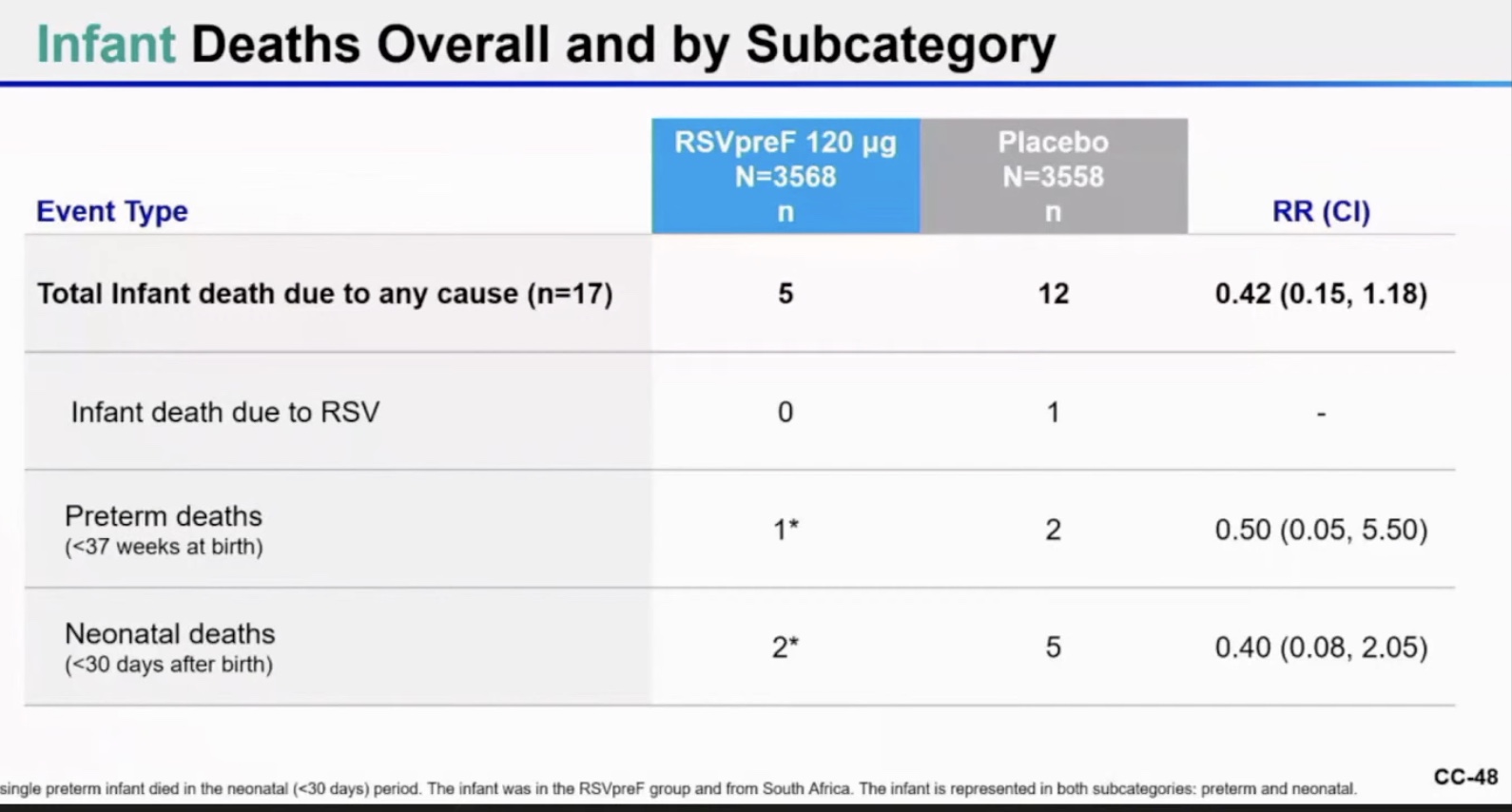
The vaccine seems to have been safe in infants, and if there were the same number of infant deaths in the vaccinated mothers’ category versus placebo category, it would look safe. But here there were 20 infant deaths in the placebo group and only 8 in the vax group. This suggests to me that the placebo group may have been deliberately selected to be a sicker or more high risk group of pregnant women to study. That would be fraud.
Given what we know about the trial sites from Brook Jackson’s evidence of the Ventavia clinical trial site, this is a reasonable question to ask. Below, the first chart is from Pfizer, which minimized it logos this time. The next two are FDA’s assessment of the Pfizer data given them.
Here is an FDA summary slide of what was known about safety. It omits eclampsia and pre-eclampsia.
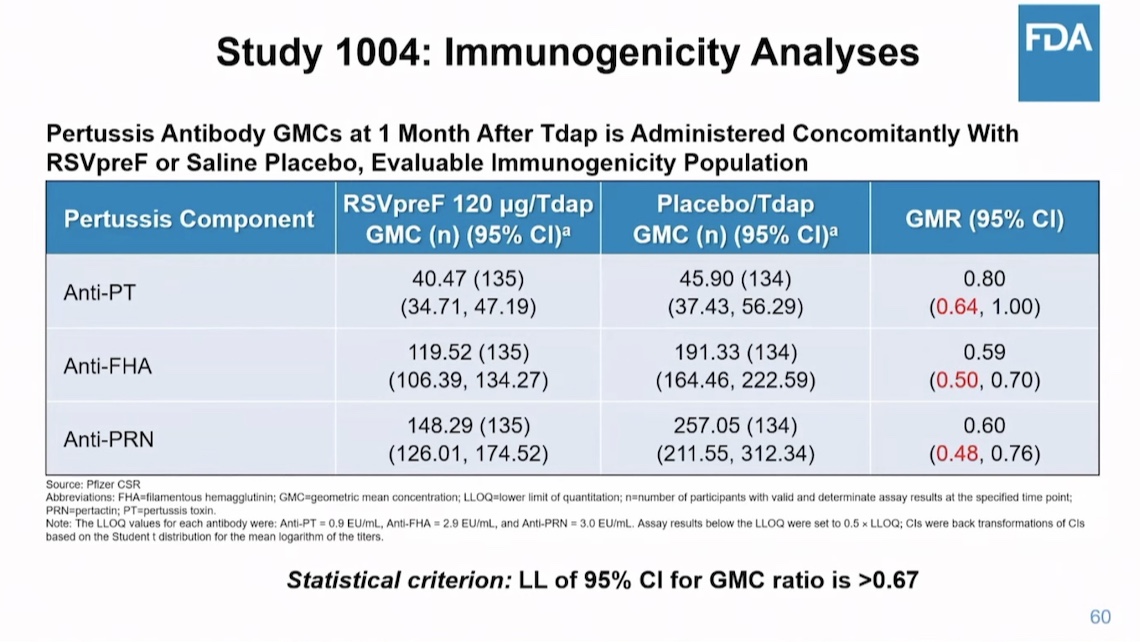
There was another point. Currently, pregnant women are advised (not by me!) to get the flu, TdaP and COVID vaccines during pregnancy. This would be a fourth pregnancy vaccine. It turned out that when the TdaP vaccine was given at the same time, the amount of antibodies produced by TdaP to 3 pertussis antigens was reduced. Pertussis is another respiratory infection that causes a similar severe syndrome as RSV and is also common. Pfizer apparently didn’t check whether this vaccine reduced the antibodies to tetanus, diphtheria, flu or COVID. So while it might reduce RSV cases, will it increase flu and pertussis cases? Here are the pertussis data:
Let me point out a couple of additional issues. The mothers were not followed long enough to see if they developed medical conditions later. One mother who developed a lot of pain after her shot was diagnosed with lupus (SLE) 9 months later.
When you are studying newborns, just because they are full term and of normal birth weight, you don’t really know whether the vaccine has caused developmental delays, cognitive effects, or motor problems that might take years to become apparent.
Did FDA stuff the meeting with 4 new temporary members in order to get the majority yes votes it wanted?
All in all, this was another disheartening day that removed yet more luster (how much is left?) from the US vaccine program and further downgraded our opinion regarding the professionalism, reliability and independence of the FDA.
Addendum: A reader pointed out that the RSV “F” antigen in this vaccine was developed at Fauci’s institute at NIH and HHS has the patent on it.