FDA Presentation on EUAs from August 2020, with my comments
Thanks for a reader for just citing this. More evidence for upcoming legal cases.
This presentation on medical countermeasures (and many others) was given by Elizabeth Sadove, JD, one of the FDA attorneys.
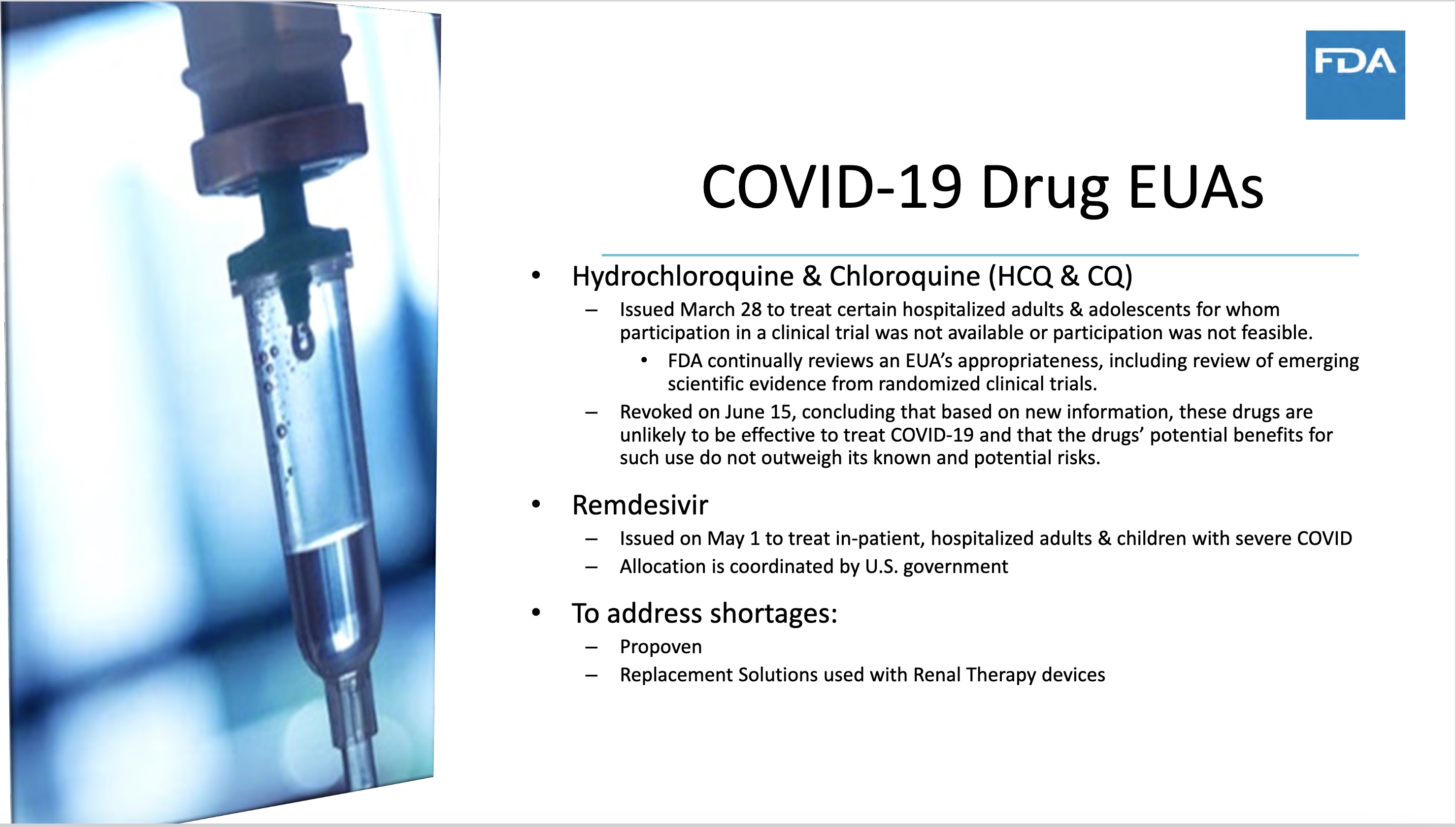
We often wonder how FDA assesses quality control of drugs and vaccines. Well, there is no quality control on this slide presentation. Ms. Sadove misspelled two of the drugs she discusses. She made the mistake of referring to hydroxychloroquine (HCQ) as hydrochloroquine. Since her office had a lot to do with issuing an EUA for HCQ in March 2020 and then revoking it in June 2020 with enormous fanfare, you would think she would know the drug’s proper name.
She could not spell the anti-swine flu drug Peramivir correctly either. In any event, here is what she and FDA had to say about EUAs:
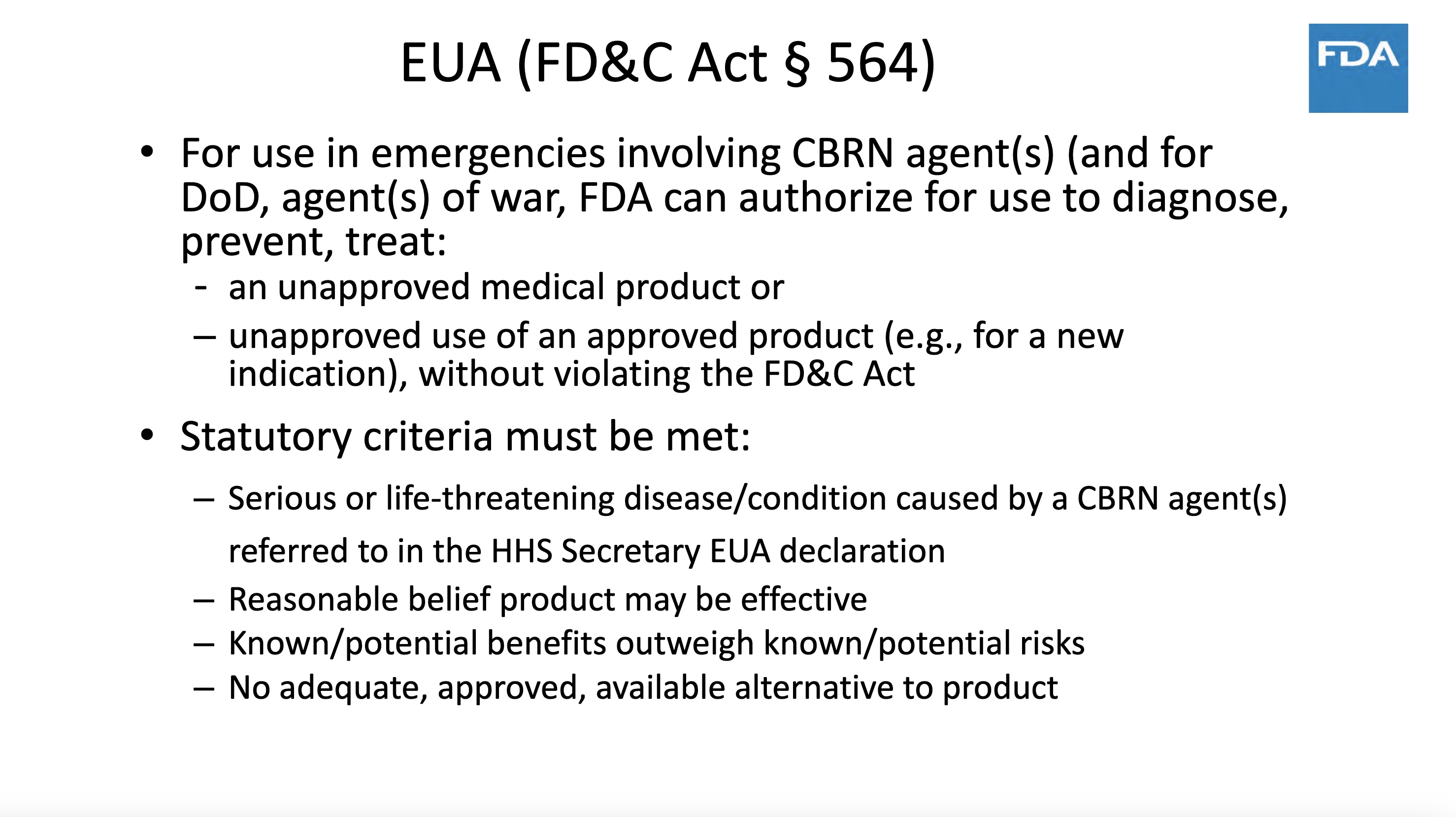
5 months into the pandemic and 570 drug development programs are in planning stage and 270 trials were reviewed by FDA. Where is the prioritization mentioned at the top of the slide?
Bullet 4 is the important one: so we can give our pharma partners and ourselves complete liability protection.
COVID is no longer life-threatening (except in rare instances) and the vaccine does not work. Risks outway benefits. There are alternative treatments.
So based on every FDA criterion there should be no outstanding EUA—and yet there is one.
cGMP refers to the requirement to meet Good Manufacturing Practices standards—which have been waived for COVID vaccines. In other words, pharma has been invited to cut corners, issue expired product, etc.
Limitations on advertising were not put in place, so that EUA product was allowed to claim safety and efficacy, even though this terminology is restricted to licensed drugs. I do not htink this was done legally.
People were illegally told they were receiving licensed product when they got EUA vials.
FDA reealized that by issuing EUAs for all these types of products, it would not be held to any regulatory standard. It was a ‘get out of jail card’ for not only the manufacturer, but also for FDA, whose employees were ‘sheltering in place’ while collecting their paychecks.
I did not mention the hydroxychloroquine scam pulled by FDA, since it had nothing to do with the Pfizer documents, but this caper involved considerable criminality also, which I detailed in earlier reports. My long story on the US and international suppression of hydroxychloroquine was most recently included in the new book, “Canary in a (Post) COVID World.” I recommend the book. I am not paid anything for my contribution, but $3 profits per book go to fund 3 top COVID organizations.
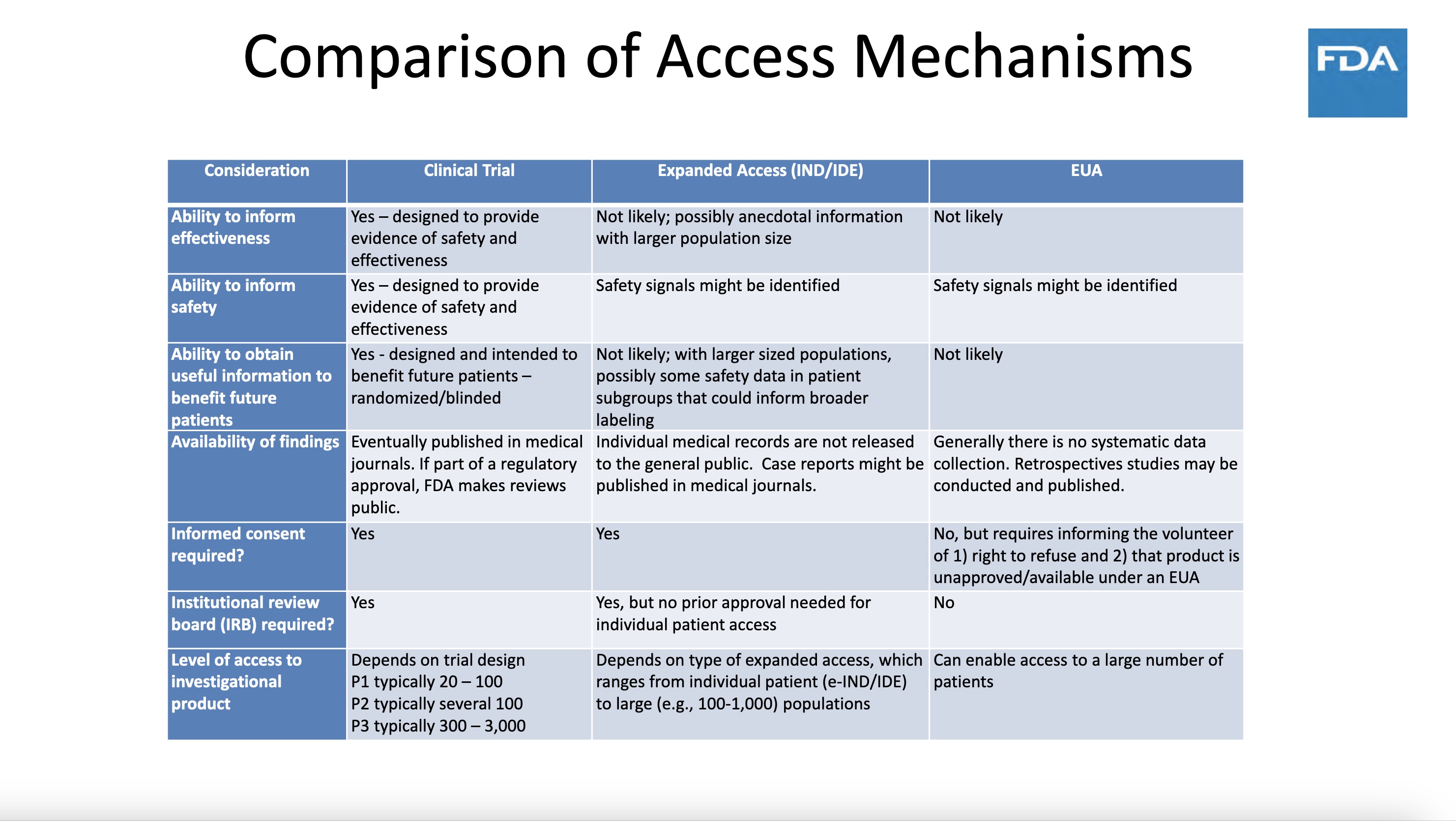
Although FDA planned to make the vaccine available “to a large number of patients” (actually not to patients but to well people in advance) it did not plan to use this mass use of EUA product (to a couple hundred million Americans or more) to assess safety and efficacy. Go figure.
Other uses of EUAs include the ability to give out what FDA otherwise terms adulterated product.
DOD used its prepositioning option during the Gulf War to create confusion over whether lots had been approved for use by FDA that were administered overseas.
During the period when there were vaccine shortages, from December 14, 2020 to April 1, 2021, FDA could have easily and ethically ordered randomized, controlled crossover studies (in which one of two matched groups gets a vaccine dose first, before its demographic was to receive it, and the matched (control) group got it later. This would have been an easy and fair way to evaluate the vaccine for safety and efficacy — but of course FDA did not do that, as its intent was to obscure the data on safety and efficacy, not illuminate it.
RECOVERY was a “simple trial” because it was predesigned to NOT collect safety information on the drugs being trialled. Probably the reason for this was to avoid identifying HCQ overdoses, and to allow the trial results for other drugs to avoid acknowledging safety problems with them.