My day in Clown World with the CDC’s Advisory Committee on Immunization Practices. On the menu: RSV, dengue, chikunguna, pneumococcus.
This morning we heard about the 4th pneumococcal vaccine on the scene.
First there was the PPSV23 (aka Pneumovax)—used for many decades. Very inflammatory. Unclear how well/if it works.
Prevnar
then surfaced about 20 years ago for kids, starting with 7 serotypes.
What it did was shift the serotype ecology, causing a reduction in
infections due to the 7 serotypes but causing an increase due to
serotypes that had not been included. So then Pfizer went to a Prevnar 13 and retired Prevnar 7. This was extremely lucrative: the kids who got Prevnar 7 were told to go back and get the Prevnar 13. Retail price $226 per dose, 4 doses needed = $904. Never let it be said that Pfizer leaves money on the table.
Pfizer’s
competitors saw dollar signs, and started mucking around with their own
pneumococcal vaccines, adding more serotypes. Merck came up with a 15
valent for $216 per dose. And today the ACIP discussed a 20 valent
pneumococcal vaccine, which has been discussed here previously. It was
called PCV20, since it has no brand name yet.
But the CDC
presented no good data on these new vaccines. Are they better than the
old ones? Worse? Better for some populations? The last time this was
discussed it totally confused everyone. So, instead of generating real
data, the CDC today rolled out—ta da! —Models. Economic models. So,
they lack data, but they don’t lack models. What they lack are reliable
data to plug into the models.
We heard about two models and I
attempted to snooze. Then the members made inane comments. Here are a
few: “Uncertainties in the efficacy remain.” “Poorly efficacious
vaccines should not be given.” “There is no efficacy data and no
corrrelates of protection.” To which one wit responded, “Our CDC
colleagues are masters at epidemiology surveillance that will give us
the answer in future.”
In other words, “we won’t know if it works unless we give it to millions of children, so what are we waiting for?”
BREAK TIME. Chairlady Grace Lee had had enough comments of that nature; you never know what will get into the newspapers.
Next was dengue.
The committee had it patiently explained again that dengue does kill
(6 Puerto Ricans a year for the past 10 years) and while one vaccine (Dengvaxia)
licensed in 2019 had already rolled out there, another was on the way.
And so we had to hear about #2 for the third time. I wrote an Op-Ed
that discussed Dengvaxia when it was licensed,
because FDA (and the EMA) licensed it after the vaccine killed a bunch
of kids in the Philippines. I really liked that Op-Ed, but it is behind
a paywall.
https://www.bangordailynews.com/2019/03/28/opinion/why-americans-dont-trust-vaccine-makers/
CDC
was conducting a war of attrition against its committee. Just like
with the PCV20, they threw models at us. Except this time, they
admitted the dengue vaccine can potentially kill—in fact, that is why
the licensed vaccine requires a lab test before it can be used. They
modelled the financial costs and benefits with and without lab tests,
for different ages, etc. Mind-numbing work, because it is based on a
bunch of guesses. Dr. Espana (the modeler) himself said that he did not
put deaths from the disease or the vaccine into the model, because they
were so rare. So—the outcome you are trying to prevent was not
included in the model. This is great stuff, CDC.
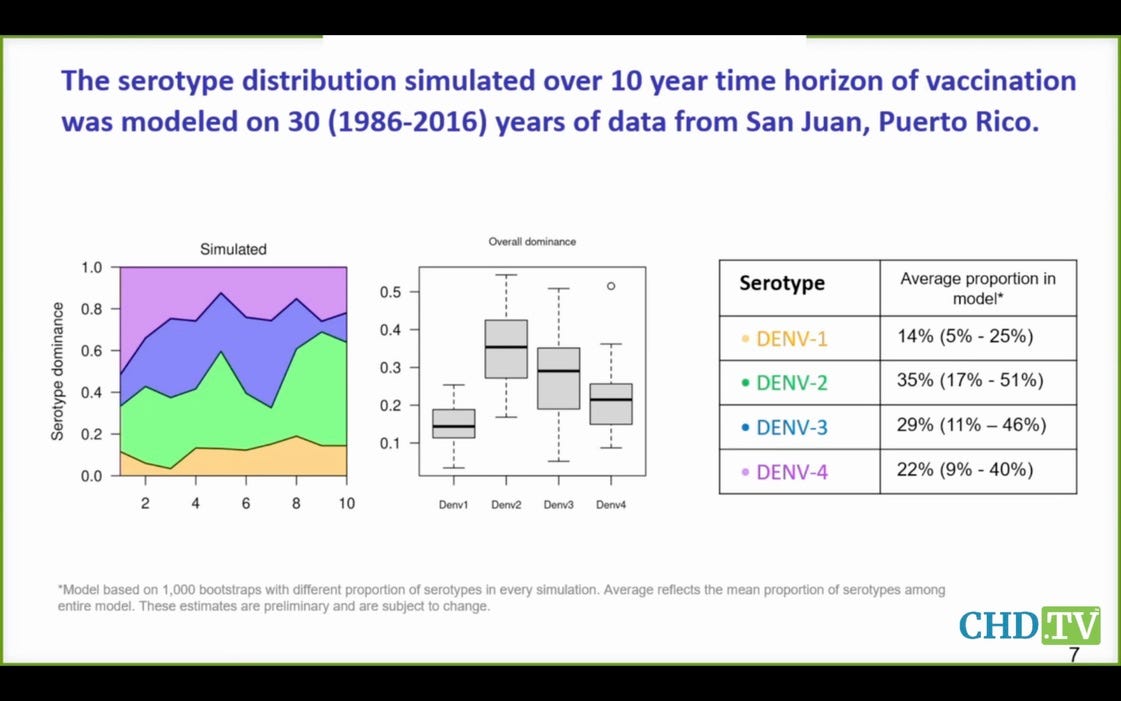
Here you see
that serotypes 3 and 4 are expected to cause half the dengue cases, but
the data suggest the Takeda vaccine may well have negative efficacy for
these serotypes. What a joke that this vaccine is even coming before
this committee—and for the third time.
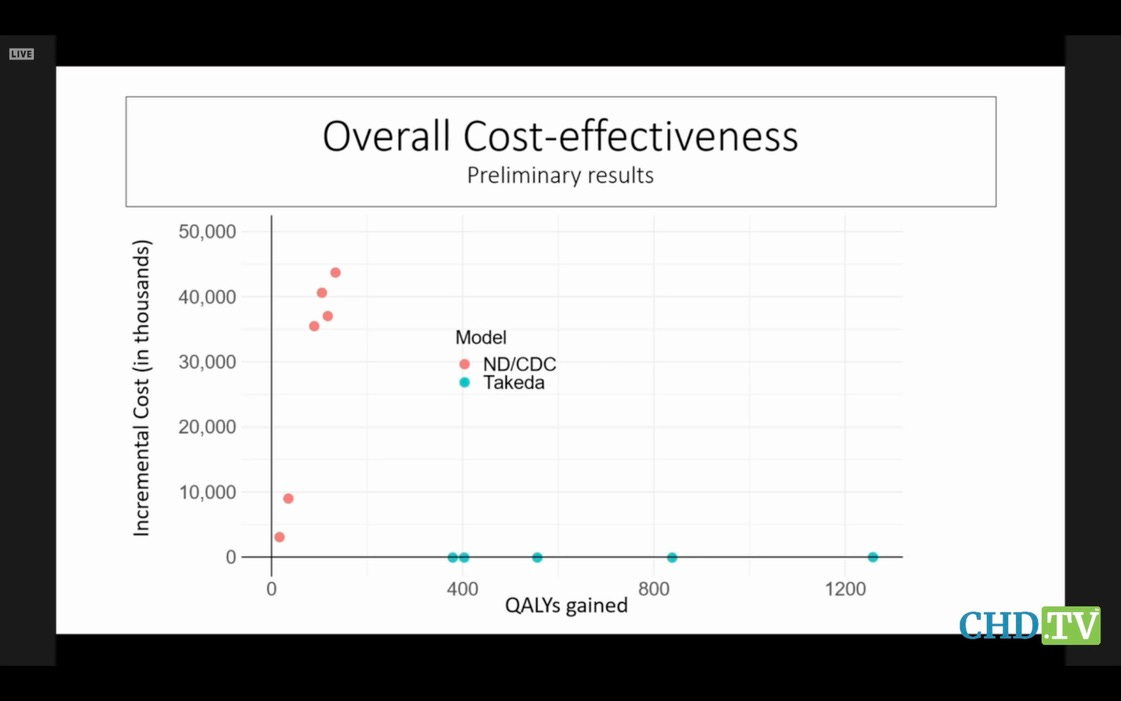
Notre
Dame was hired by CDC to create a model, and Takeda (the mfr) created
its own model. Unsurprisingly, their predictions were extremely
different. But no one on the committee dared hint that the emperor had
no clothes, and no one asked why CDC was wasting everyone’s time with
this nonsense. Here are their models’ results, compared. Hope you get a
belly laugh:
It’s
a war of attrition. And the committee will be mezmerized by the
details of the trees and forget the forest when this stupid vaccine
finally comes up for a vote.
Today’s slides were very simplistic but they do convey the flavor of the materials presented.
So after being left hanging with these 2 vaccines it was time for lunch.
Now for chikungunya.
We have also heard about this one previously—we have heard about all
of them previously. The idea seems to be to present the bad news, but
wait a few months for a vote until the members have forgotten it.
One
case of chikungunya is reported in lab workers in the US every 2 years,
but Paraguay is just getting over a massive outbreak with over 160,000
reported cases. Also cases in Argentina, Brazil and Uruguay. This is a
mosquito-borne virus, like dengue. It has been studied in military
labs, at a BSL-3 level. The vaccine is being regulated under the “Animal Rule”—pretty
bizarre when Paraguay provided the perfect laboratory to study the
vaccine—which means no efficacy testing needed, just show us a few
antibodies and give it to a few folks, watch em for a bit, and call that
safety testing.
Or maybe FDA did require a study in
Paraguay, but if the results were not to someone’s liking so they are
being hidden—like CDC did with its monkeypox trial in the Congo.
Tomorrow we hear more about monkeypox vaccine, can’t wait.
Why in
heaven’s name are we doing this? Licensing a vaccine without data for a
disease that does not exist in the US. Don’t know but it is creepy.
And the animal rule depends on there being very few cases, but 160,000
cases should have mooted its application. Evidence of more FDA
malfeasance:
https://www.fda.gov/drugs/nda-and-bla-approvals/animal-rule-approvals
The regulations commonly known as the Animal Rule
(21 CFR 314.600-650 for drugs; 21 CFR 601.90-95 for biologics;
effective July 1, 2002) allow for the approval of drugs and licensure of
biological products when human efficacy studies are not ethical and field trials to study the effectiveness of drugs or biological products are not feasible.
Now for RSV:
the pregnancy vaccine and the monoclonal. This sickens me. We heard
about each, and we heard a lot about modelling the economics if one or
both were used together. The models are admittedly worthless, except
now we are playing with the lives of pregnant women and newborns. GSK
admitted their vax caused premature births, but Pfizer just got its
nearly identical vax licensed. GSK was on the line, along with Pfizer,
to give its rival a black eye if it got the chance.
Here the modeller admits his model aint worth much.
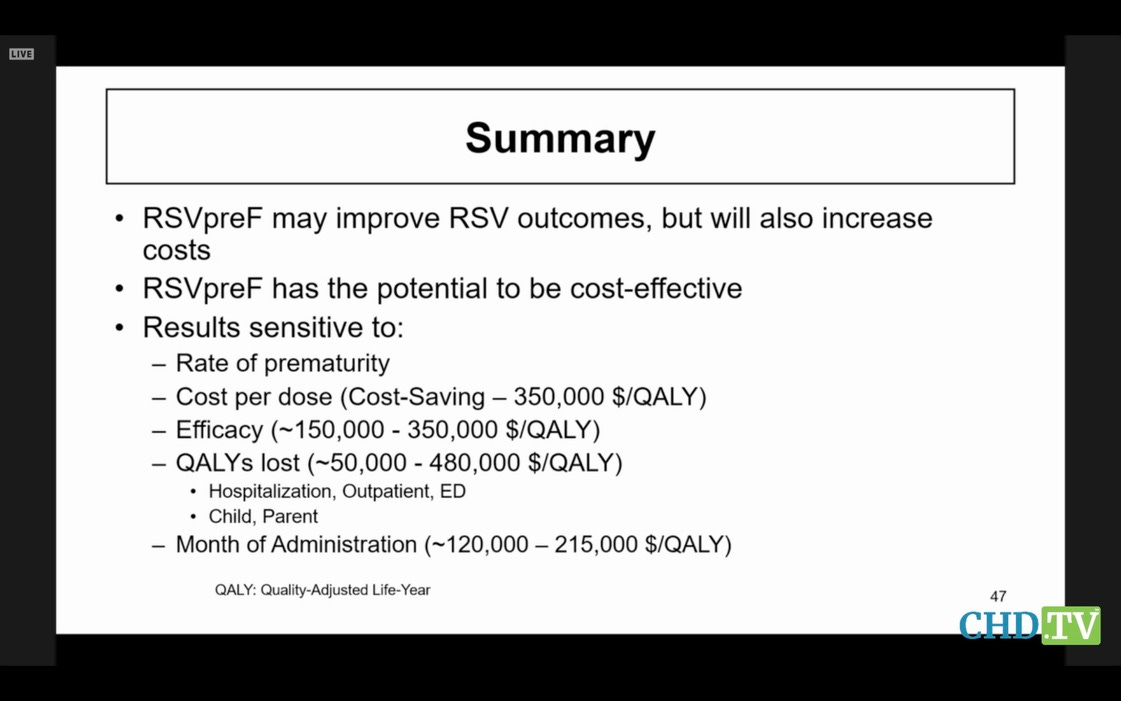

Instead
of being horrified at the potential costs, and the fact that GSK
wouldn’t have given up its vax at the end of the race unless it was
convinced it caused premature labor, the committee tried its best to
make light of the prematurity issue. SURELY CDC could find some way to
help them justify its approval? Surely CDC’s modellers and its spin
doctors could make that nagging premature labor go away, so our ACIP
members would not get caught with their pants down later for having
okayed it?
Then they got onto another track: who was going to
pay for the vaccine and the monoclonal? OMG, if we can’t hang the costs
on Uncle Sam, what then? Do you expect us to give this out in our
offices and not get reimbursed? Patients don’t pay cash for vaccines.
Members attempted to direct CDC to put the vaccine and monoclonal onto
the childhood schedule or the Vaccines for Children program. They
attempted to tell CDC that nirsevimab, a therapy, should be billed like a
vaccine. The appropriate CDC offical had to explain very politely that
they have no jurisdiction to tell Uncle what program to put his
vaccines or monoclonals into.
Of course they should have had no
worries—someone else will take care of those details. But it was
striking to me how involved they were in the financing of the products,
which together cost $700. And why the high cost didn’t seem to bother
them at all.
And then I remembered. Pediatricians sink or swim on
the vaccines they administer. $400 bonuses for each fully vaccinated
child in the practice. Higher bonuses if a higher percentage of the
patients are fully vaccinated. Low reimbursements for other billable
activities. If a pediatric practice does not give out loads of
vaccines, it will simply go under, unless it has a unique specialty
practice and does not bill insurance.
Therefore, how can you expect a pediatrician to look closely at vaccines? It would be like asking him/her to dig his own grave.
And then they voted YES for the 4th pneumococcal vaccine, and I said a prayer that it was finally over.